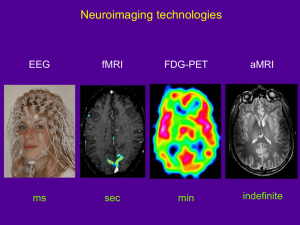
Visual Stimulation in the MRI Lab
advertisement

Generic Risk Assessment Form IMPORTANT: Before carrying out the assessment, read the Guidance Notes provided on this website. Department School of Psychology Building CUBRIC Room No Name of Assessor 30 April 2007 ID No [optional*] Dr S K Rushton Date of Original Assessment MRI Suite * You may wish to use an ID No. if you want to have a unique identifier of the risk assessments within your Department Brief Description of Procedure/Activity and its Location: [Guidance note 2] Perception of object movement during simulated self-movement in normal healthy research volunteers in the MRI system at CUBRIC The Magnetic Resonance Imaging system (MRI) system is housed within the MRI suite of the CUBRIC building. The system can be used for research scanning of brain activity in human participants. MRI scanning of participants is included in a separate risk assessment. During MRI scanning of participants in some situations visual stimulation will be applied to participants. Visual stimuli can be presented on either a back projector screen or a goggles system, both in the MRI suite. This risk assessment covers visual stimulation of participants in the MRI system. As per the MRI scanning risk assessment a minimum of two trained Cardiff University staff or students will be present for each scan, at least one of whom is a full member of staff. This document covers MRI scanning by Cardiff University staff only. Note: Should no potential hazard be identified as associated with the procedure/activity in this location, you may stop the risk assessment at this point [See Guidance note 2] __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 1 Hazards Involved: [Guidance note 3-5] Substance/Item of equipment/procedure or physical location Visual stimulation of normal MRI participants via backprojection screen or goggles. Visual stimulation of normal MRI participants via backprojection screen or goggles. Associated hazards For a small proportion of the epileptic stimuli commonly used in laboratory situations (e.g. checkerboards and gratings) can induce photosensitive seizures Migraines can be induced in some people by flickering or high contrast visual stimuli. We use full-field motion stimuli and these could produce sensations of nausea in susceptible observers. Existing Control Measures 1. During the participant screening for MRI scanning it is determined whether potential participants have any history of epilepsy or light-induced seizures. Volunteers with epilepsy or a history of light-induced seizures are excluded from participation. Severity Likelihood Risk 1-4 2 4 1. On the volunteer information sheets given 1-3 to participants before undergoing MRI scanning they are informed of the potential risk of migraine. Participants can choose at this point whether they still wish to take part in the study. 2. Participants are always instructed as per ethical guidelines that they can cease an experiment whenever they choose (if they were to start to develop a migraine). 2 3 1 1 Observers instructed to press squeeze-ball if they feel nausea developing. Study will be terminated. __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 1 2 __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 3 Persons Potentially at risk: (Guidance note 6) Research volunteers during MRI scanning. Additional Control measures which will need to be applied to reduce the risk to an acceptable level (Guidance note 4) Control measure Date of Implemented Implementation by: Remaining Level of Risk Because the risk level is within the acceptable level no further action need be taken Action in event of an accident or emergency (Guidance note 7) The suite will be evacuated as appropriate. CUBRIC First-aiders and/or a 999 call will be used if needed. Arrangements for Monitoring Effectiveness of Control: (Guidance note 8) __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 4 Periodic review by CUBRIC Management Receipt of the Risk Assessment: (Guidance note 9) This assessment has been issued to and read by: Name of Recipient: Date of Receipt Signature __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 5 Review of the Risk Assessment: (Guidance note 10) Have the control measures been effective in controlling the risk? Yes No Have there been any changes in the procedure or in information available which affect the estimated level of risk from the listed substances? Yes No What changes to the control measures are required? Date of Review: Date of Next Review Name of Reviewer Signature: __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 6