An MEG/anatomical MRI study
advertisement

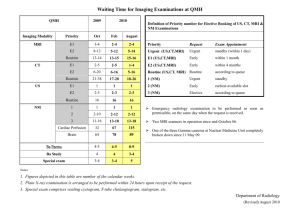
Generic Risk Assessment Form Department School of Psychology Building CUBRIC Room No Name of Assessor Date of Original Assessment 30 April 2007 ID No [optional*] Dr S K Rushton MRI Suite You may wish to use an ID No. if you want to have a unique identifier of the risk assessments within your department Brief Description of Procedure/Activity and its Location: [Guidance note 2] Perception of object movement during simulated self-movement in normal healthy research volunteers in the MRI system at CUBRIC The magnetic resonance imaging (MRI) system is housed within the MRI suite of the CUBRIC building. The system can be used for research scanning of tissue structure and function in human participants. This risk assessment covers the scanning of normal healthy volunteers. A minimum of one trained CUBRIC staff member and one additional Approved User is needed to be present for each scan. For the initial core-staff, scan training will be provided by the MRI supplier, General Electric. After this initial period, training will be provided by CUBRIC staff. Note that this document covers MRI scanning by Cardiff University staff only. CUBRIC is a new facility and the risk calculations detailed on the next two pages are made assuming the control measures detailed in the tables on Page 1 and 2 are properly implemented. __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 1 Hazards Involved: [Guidance note 3-5] Substance/Item of equipment/procedure or physical location Associated hazards Static magnetic field produced by the superconducting magnet of the 3T MRI machine 1. Projectile action of loose objects containing ferromagnetic metals (such as iron, steel and nickel) brought within the vicinity of the magnet. 2.Electrically, mechanically or magnetically activated implants (such as cardiac pacemakers) may be subject to electromagnetic interference, heating and a mechanical force in or close to the MR system 3.Ferro-magnetic prostheses and implants may be displaced when introduced to the magnetic field. Metallic implants may undergo heating during a scan as a result of RF exposure. 4.Metallic foreign bodies from working with metal or shrapnel wounds Existing Control Measures Operators and researchers are instructed to follow the CUBRIC Rules and Procedures Document to ensure the safety of themselves, colleagues and participants. Only subjects who have been screened may enter the controlled area. This includes all accessible areas where the magnetic field exceeds 0.5mT(5 gauss) Severity 5 Likelihood 1 Risk 5 All equipment to be used within the control area must be tested for ferromagnetic components. Equipment that is safe to use in the MRI suite must be clearly labelled. The MR suite is equipped with non ferrous carbon dioxide fire extinguishers. All Authorised users must be 1. Qualified in first-aid to a minimum for appointed persons; 2. Have passed a MRI safety test; and 3. Hve read the MDA guidelines and the CUBRIC Rules and Procedures document. Authorisation to be granted by the CUBRIC Operational Group (COG). Participants must complete a two tiered screening form to determine if it is safe for them to be scanned. Maintenance & cleaning staff are not permitted to enter the Inner Controlled area. Warning signs are posted on the entrances to the MRI __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 2 suite the signs to indicate implants that can be affected by a magnetic field e.g. cardiac pacemakers, hearing aids. The inner controlled area is marked as a ‘Red Zone’ and it is indicated that a ‘Permit to Work is required’ Reaction of participants during scanning. Claustrophobia Any form of discomfort during scanning e.g. stiffness. Heating from tattoos that contain metallic content. Heating from make up. The extent of the 5 Gauss line has been provided by a third party (MagNET, UK). A hand-held alarm and panic button is 3 provided, which is tested at the start of each scan session. 1 3 1 5 All participants are informed that they may withdraw from an experiment at any time without giving a reason. An intercom is provided between the operator and the participant for hearing participants. A projection screen is available for communication with deaf participants. Participants with tattoos will be advised to report immediately any discomfort. Participants are asked to remove make-up before scanning. Emergency Procedures relating to Fire. Fire in MRI suite, Appendix 6 of the CUBRIC Rules and 5 Procedures document details the evacuation procedure to be followed OSHEU have a ‘Special Risks Register’ which includes the MR unit. The Fire Service has access to this register, and has been invited to make an 11D (Special Risk Familiarization) visit. __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 3 Liquid Helium surrounding the main magnet of the MR system Escape of Helium can cause asphyxiation from the reduction of oxygen in the air. Contact with helium can cause cold burns, frostbite and hypothermia. Helium dropping below 50% of the capacity could cause the magnet to fail and a possible quench (see risk assessment for quench). The equipment is under maintenance contract 5 and handling of cryogens to be carried out by 1 5 1 2 General Electric and their subcontractors. The helium for the MRI is replenished during routine servicing by General Electric. Air conditioning equipment to be monitored and maintained by Estates. Provision of an oxygen monitor inside the control area that activates an alarm should oxygen level drop below safe minimums. There is a 600 mm x 600 mm pressure equalizing waveguide vent mounted in the RF ceiling. An open grille is fitted in the suspended ceiling to allow air to move in and out of the room if the pressure changes. There is an emergency extract fan which is part of the scanner room ventilation design and it can be switched on manually or triggered automatically by the oxygen monitor and this provides an extraction rate of 1200 cfm. The oxygen monitor is in the control room but the sensor is on the ceiling above the magnet. Authorised users must have read the MDA guidelines for MR equipment and the CUBRIC Local Rules and Procedures document before operating scanning equipment. Use of laser localiser during participant positioning before scanning Damage could potentially occur to eyes if looking straight in to the beam, or Participants will be asked to close their eyes 2 during laser positioning. __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 4 if beam is deflected into the eyes. Blink response exposure. protects from accidental Each laser light localizer has an output of less than 1 mW at a wavelength of approx 650 nm. Operators are to be aware of and minimize the risk of deflecting beam into their eyes. These are classed as class II lasers. Exposure may cause dapple affect Scanning participants Person taken ill or injured in scanner (unrelated to static magnetic field) Screening forms should eliminate anyone 5 known to be at risk. When necessary, the GE scanner couch can be undocked – and can be wheeled out of the inner controlled area. Emergency services, if necessary, will be called (999) All approved users will have been trained in basic first aid (to ‘appointed person’ stage) 1 5 Scanning participants Person injured in scanner due to projectile effect Screening forms should eliminate chance of 5 potential projectiles being taken in the vicinity of the magnetic field. If necessary, the GE scanner couch can be undocked – and can be wheeled out of the inner controlled area. Emergency services, if necessary, will be called (999) All approved users will have been trained in basic first aid (to ‘appointed person’ stage). If it is impossible to remove the metallic object (which is preventing participant from being removed from scanner), a quench can be initiated (but see separate risk assessment for 1 5 __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 5 quench) Quench of the magnet (manually initiated or spontaneous) Helium dropping below 50% of the capacity could cause the magnet to fail and a possible quench). A quench could also arise due to malfunction of the equipment or ferromagnetic objects being attracted to the scanner. The magnetic coil windings can touch the inside of the scanner causing the magnet to become partially resistive and start to generate heat. One litre of helium equals 750 litres of gas. In a quench typically, 60% of the liquid helium will change to a gaseous state. This results in approx 750,000 l of gas that is vented within 20 sec. A Quench is only performed if the emergency 5 services need to gain access to the controlled area or if a participant is in a life threatening situation due to the magnetic field. 1 5 Only MRI safe or MRI compatible equipment is allowed in the examination room. All equipment to be marked. All new equipment to be taken into the examination room must be tested and approved by the MRI Director. All system faults will be reported and documented in the Incident Book, which is kept in the control room, If the fault prevents normal safe operation the room will be signed out of use and a sign fixed to the equipment. The equipment must be signed over to the engineer and back after it has been repaired and tested.The oxygen monitor will sound if oxygen levels become low. Damage to the quench pipe may cause the following during rapid boiloff: __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 6 Excessively cooled gases will freeze water molecules in the area adjacent to the magnet, causing a dense white fog. Oxygen in the room will be displaced by helium, making it difficult to breathe. The helium gas that escapes during the quench is extremely cold and may freeze objects in its path. Positioning participant for the scan MR phantoms. Used for setting up MRI and before placing a participant in the scanner. Incorrect positioning of participants in scanner can create conductive loops due to the Radiofrequency magnetic fields. RF burns can occur where the arms and thighs meet. Fluid leaks in case of improper use, damage or during refilling with distilled water. Some phantoms can contain nickel sulphate which can cause skin allergies in sensitive persons and possible carcinogenic affects. Ensure that there is no skin to skin contact and 1 use pads between limbs. Ensure that the participant's skin does not touch the bore of the magnet and use MR compatible foam pads if necessary. 1 1 Phantoms must be stored and used at 1 temperatures from 0 C to 50C. 1 1 Eating and drinking are not permitted when working with MR phantom fluids. Hands must be thoroughly washed with soap and water after working with phantom fluids. __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 7 Top up with distilled water to be carried out by GE engineers under the maintenance contract. __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 8 Persons Potentially at risk: (Guidance note 6) Participants and staff during MRI scanning. Additional Control measures which will need to be applied to reduce the risk to an acceptable level (Guidance note 4) Control measure Date of Implementation Implemented by: Remaining Level of Risk As this is a new facility and procedure, the Control measures and the level of Risk assessed (assuming compliance with those control measures) are listed on Pages 2-3. At this stage no new control measures are therefore needed. Action in event of an accident or emergency (Guidance note 7) The suite will be evacuated as appropriate. CUBRIC First-aiders and/or a 999 call will be used if needed. Arrangements for Monitoring Effectiveness of Control: (Guidance note 8) Periodic review by CUBRIC Management __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 9 Receipt of the Risk Assessment: (Guidance note 9) This assessment has been issued to and read by: Name of Recipient Signature Date of Receipt __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 10 __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 11 Review of the Risk Assessment: (Guidance note 10) NOT APPROPRIATE AS YET – SINCE THIS IS A NEW RISK ASSESSMENT. Have the control measures been effective in controlling the risk? Yes No Have there been any changes in the procedure or in information available which affect the estimated level of risk from the listed substances? Yes No What changes to the control measures are required? Date of Name of Reviewer Review: Date of Next Review 4th July 2009 Signature: __________________________________________________________________________________________________ Occupational Safety, Health and Environment Unit/September 2004/RISKASS Form 12