eScholarShare - Drake University
advertisement

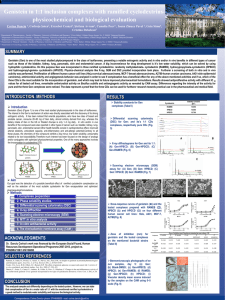
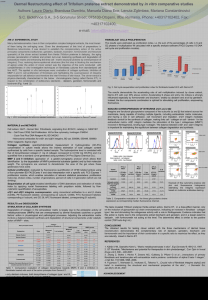
Analysis of Epigenetic Effects of Genistein on Cancer Cells in Culture Jonathon Zolkoske Drake University College of Pharmacy and Health Sciences Abstract: The overall objective of our research project is to analyze the anti-proliferative effects of genistein, an isoflavonoid found in soybeans. Soy products, including our compound genistein, have received new marketability as a health supplement because of their antioxidative properties. Recent studies have documented other biological effects of genistein including anti-carcinogenic effects on certain types of cancers. Mechanisms of such anti-proliferative effects have not been fully elucidated. Specifically the role of epigenetic mechanisms in the anti-neoplastic activity of genistein is poorly defined. This project explores the effect of genistein on acetylation/deacetylation histones in two breast cancer cell lines. Such analysis is anticipated to aid future pharmacotherapeutic applications of genistein. However, it is still unclear if genistein expresses its epigenetic modifications independently of the estrogen receptor pathway. We hypothesize that genistein produces its effects via an epigenetic mechanism in an estrogen receptor dependent/independent model. Clarifying the molecular mechanism by which genistein acts epigenetically will increase its potential use as an anti-cancer pharmacotherapy. Therefore, we will be evaluating the histone modifications, specifically acetylation/deacetylation, of genistein treated HTB-19(ER- negative) and HTB-20 (ERpositive) breast cancer cells using an anti-acetyl lysine antibody in order to detect acetylated histones. Introduction: Genistein is a naturally occurring isoflavonoid, a class of biologically active plant metabolites, which are commonly found in legumes such as peas, peanuts, soybeans, etc2. These legumes are known to have very high concentrations of isoflavones like genistein and daidzein2. Isoflavones act as phyto-estrogens being that they are similar in structure and function of the endogenous 17-β estradiol steroid hormone11. Isoflavones have received considerable attention by researchers and nutritional consumers because of the potential health benefits (anti-oxidant and anti-inflammatory) of genistein and daidzein. Epidemiological studies have researched the correlation between lower incidence of heart disease, various cancers, and other chronic diseases compared to the high dietary intake of soy products within the East and Southeast Asian populations8. Follow up epidemiological studies also revealed that this lower incidence of carcinoma was seen in immigrant generations of the same Asian communities that still had high dietary intake of isoflavones like genistein8. These studies suggest that genistein may affect the incidence of breast cancer by lifestyle and not genetic factors. This is why we look to an epigenetic mechanism that includes different epigenetic modifications to DNA and chromatin with or without interaction of the estrogen receptor pathway Introduction (continued): Protein Extraction Stored samples were taken out of -80°C freezer and placed to thaw on ice. Cell pellets were suspended in 250-500uL of Tissue-Protein Extraction Reagent (T-PER) containing 2 protease and 1 phosphatase inhibitors (ratio of 1uL of inhibitor per 100uL of T-PER). This mixture was vortexed several times to fully suspend cell pellets in solution and then centrifuged at 14,000rpm for 10 min at 4°C. The supernatant was pippetted off into 3 separate aliquots (25uL) and stored at -80°C degrees. The cell pellet was also conserved and stored separately at -80°C degrees. Bradford Protein Assay In research studies genistein has been found to be bi-phasic meaning that at low concentrations it exerts estrogenic effects and at high concentrations it displays an antiestrogenic effect12. Genistein is of interest at high concentrations due to its antiproliferative effects on certain carcinomas. Some of the different modes of inhibition at the cellular level include inhibition of cell proliferation, induction of apoptosis, induction of differentiation, and modulation of cell cycle progression1. Inhibition also occurs on the enzymatic level. Enzymatic level inhibition affects the activity of protein tyrosine kinase, topoisomerase, aromatase, and 17b-hydroxysteroid oxidoreductase1. These antineoplastic effects are of interest in current and future work with breast cancer cells. In our current study with HTB-19/HTB-20 breast cancer cells we are examining the antiproliferative effects that genistein may be inducing via chromatin remodeling, specifically acetylation/deacetylation of histones. It is generally known that acetylation of histone lysine residues facilitates transcriptional activation and induction of gene expression. In a recent study by Majid et al. it was found that BTG3, a tumor suppressor gene, was epigenetically silenced and reactivated by genistein induced promoter demethylation and active histone modification in two prostate cancer cell lines14. We wish to analyze these epigenetic modifications using a similar model with genistein treatments but with a different cancerous cell line (HTB-19, ER-negative) and (HTB-20, ER-positive). Materials and Methods: Cell Culture Human breast carcinoma cell lines (HTB-19, HTB-20) were cultured in RPMI medium supplemented with 10% fetal bovine serum, L-glutamine, penicillin, and maintained in an incubator with a humidified atmosphere of 95% air and 5% CO2 at 37C. Both cell lines were subcultured under sterile conditions washing with PBS (2x) and trypsinization (~3-4 min) to cleave proteins with strong interactions to the flask. Subculture of cells occurs after 80-90% confluent growth and cells are re-suspended in fresh media and stored in the incubator. This process was repeated until total cell count had reached optimal number (~95 – 100 million cells) to complete genistein treatment experiments. Genistein Treatment Many biological effects of genistein are mediated through the biochemical pathway normally used by estrogen. Initially, estrogen (E) binds to a specific receptor protein (R) in the cytoplasm of a target cell. The E-R complex then moves into the nucleus and activates or inactivates expression of specific target genes. This, in turn, modulates the specific pathways that the target gene products may be involved in by either up regulating or down regulating of protein formation and altered cellular response. When genistein (G) enters the cell (e.g. via dietary intake), it competes with E for binding to R. The normal E-R pathway is disrupted and the normal cellular functions are affected. Many well-documented pre-pubertal side effects of genistein are attributed to this ability of genistein to disrupt normal physiological processes, giving it the title of an ‘endocrine disruptor’. On the other hand, in the absence of E (such as in menopausal women), genistein may act as a substitute for E and carry out some of the normal functions of E. This forms the basis for advocating genistein as a natural substitute to treat postmenopausal complications8. Materials and Methods (continued): In order to study the effects of genistein on both (HTB-19, HTB-20) cell lines as a function of concentration and time the cell cultures were harvested by usual washing with PBS and trypsinization under sterile conditions. The cells were counted to ensure a minimum of 300,000 cells/mL was reached to proceed with the experiment. Cells were distributed in 50 mL volumes to 6 separate flasks (150cm2) and 10 mL of cells was distributed to a 75cm2 flask for a control. 100uL of DMSO was added to the control flask (75cm2) to be a vehicle control and placed in the incubator for the entirety of the 60 min experiment. The 6 150cm2 flasks (labeled A-F) were treated with 500uL of genistein at different concentrations (10mM, 5mM, 2.5mM, 1mM, 100uM, and 10uM). The flasks were gently mixed after adding each concentration of genistein. Immediately after adding the genistein concentrations, 10mL of the cell suspension was removed and pipette into a pre-labeled (A1-F1) 15mL conical tube. This process of genistein treatment at previously mentioned concentrations and removal of 10mL samples from each flask was done at time points 10’, 20’, 30’, and 60’. All samples (A1-A5, F1-F5) were placed on ice under non-sterile conditions. Samples were then centrifuged at 5,000rpm for 7min with the supernatant being discarded. Two ice cold PBS washes (10mL) were completed by adding PBS to each sample and repeated centrifugation (5,000rpm for 7min) with the supernatant being discarded. Pellets were stored at -80°C. A series of dilutions of the protein samples were prepared at ratios of 1:1 and 1:5. The dilutions were analyzed using the Bradford micro-assay which uses dye-binding method to measure absorbance of protein containing solution at 595nm in the spectrophotometer. The absorption values were recorded to calculate the end protein concentration in each sample solution. Bovine serum albumin (BSA) was used as a standard and produced a standard curve for protein concentration with absorbance readings at 595nm. The standard curve was constructed by using known BSA concentration dilutions from a working solution of 1ug/1uL. The graph shows the value of the slope to be the amount of protein in each sample and the accuracy is determined by the r2 value (>.98). The protein amounts calculated from this assay can be used to analyze equal amounts of protein in each sample for sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and Western Blot. SDS –PAGE Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis Appropriate volumes of each protein extraction were diluted so equal amounts of protein will be loaded into each well of the gel. Protein samples were combined with an equal volume of 2x SDS-sample buffer containing 2% SDS and 1 M β-mercaptoethanol as the reducing agent. Each sample mixture was vortexed and quickly spun before incubation at 95°C for six minutes. The samples were quickly spun again after heating and were loaded onto a 4-20% Tris-glycine gel. Along with the protein samples from the treated cells other controls were also load onto the gel to provide a negative and positive control for comparison. The gel apparatus was filled with 1% Tris-glycine running buffer and electrophoresis was carried out at 125 volts for 90 minutes. The gel was removed from the plastic cassette and stained (coomassie brilliant blue) and destained or transferred on to a PVDF membrane using the Western transfer. Western Blot Immediately following the electrophoresis, the gel was washed with Tris-glycine transfer buffer. Sponge pads were soaked in cold ( 4oC) 1x transfer buffer until the assembly of the transfer module. Two filter papers were briefly rinsed in water and 1x transfer buffer. The PVDF membrane is submerged in methanol before soaking in water and transfer buffer due to its hydrophobic properties. The transfer module is assembled in an ice bin by placing three sponge pads in the box followed by a filter paper and the gel. The PVDF membrane was placed on the gel carefully making sure that there were no air bubbles between any of the layers to disrupt the flow of current and ultimately the transfer of proteins. A corner is a cut from the membrane in order to distinguish which side the protein will be transferred. The second filter paper was placed on the membrane followed by three to five more sponge pads. The module was closed and placed in the running apparatus where the inner chamber was filled with the 1x transfer buffer until almost full and the outer chamber was filled with cold distilled water (2/3 full). The cables were attached to the power supply and the apparatus and the transfer was run at 25 volts for 150 minutes. Following the transfer of protein to the membrane, the blot is soaked in a non-specific blocking agent, Blotto in tris-buffered saline with tween (TBST), to saturate the areas on the blot where there is no protein so only specific binding happens through the detection process after the blot is soaked for at least 4-5 hours. Primary antibodies for the target protein were diluted to 1:1000 (5uL of antibody and 5mL of blotto). The membrane was exposed to the primary antibody solution for an hour. The blot is then washed 3 times each time for 10 minutes in TBST. The blot is then exposed to the secondary antibody in another 1:1000 dilution. After one hour incubation with the secondary antibody, the blot is washed 3 times each time for 10 minutes in TBST. Presence of the antigen can be detected using chemi-luminesence. After the washing after the secondary antibody a luminescence solution was carefully dropped onto the blot. The solution was prepared by adding 25μL of solution B to 1mL of solution A that are provided in the GE ECL kit. The blot was then placed on a glass screen so the imager could expose the blot and analyze the bands after exposure. Results and Discussion: Following genistein treatment and protein extraction of both cell lines (HTB-19 further analysis was done to determine what amount of protein within each sam (A1/A5 – F1/F5). Bradford assay resulted in a strong standard curve (r However, the Bradford assay calculations done for each treatment group indica low amounts of protein. These calculations were validated following SD (Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis) analysis. Afte SDS-PAGE analysis with HTB-19 and HTB-20 samples (A1-A5/F1-F5 wit control) it was determined that there was a very minimal amount of protein o after staining with Coomassie brilliant blue. The results were confirmed with SDS-PAGE gels of different samples producing very similar results. The contro low protein expression on the gel as well. It was determined to not cont Western Blot protocol for these samples following low protein expression af PAGE analysis. Western Blot protocol was done for known samples (liver) an with actin antibody and anti-actin secondary antibody. The protein detection sensitive process that must to be carried out carefully to allow the antibodies the specific sites on the membrane. This Western Blot protocol was done for v of technique and protocol. The analysis of these treated samples revealed a loss of mass amounts protei during experimentation; however, the question of when and where experimentation remained unanswered. To answer this question many other st analyzed in order to identify possible faulty protocol. First, the stain (coomaiss blue) was in question. A new stain was made using 1% coomaisse brilliant adding acetic acid, methanol, and deionized water in their respective amounts. SDS-PAGE analysis was performed with known samples (liver) and genistei samples along with a control and marker. This resulted in similar protein expr the treated samples on the gel, but the known samples as well as marke noticeable increase in protein expression. Finally, the genistein treated samp that were conserved after T-PER extraction were also analyzed due to the pos incomplete extraction. The pellets from samples F1-F5 were run on a gel us PAGE analysis with known samples (liver), a control, and a marker (SeeBlu hypothesized that the loss of protein or cells could have been following the tre genistein experiment during PBS (Phosphate Buffer solution) washes and cent prior to storage at -80°C. Another potential explanation for the decreased a protein in the genistein treated samples could be due to the overestimation during culture and prior to genistein treatment experiments. After encountering a few extra problems throughout this process, it didn’t allo time to complete further genistein treatment experiments in order compile com results from the HTB-19/HTB-20 cell lines. The next step in this project is to co grow and harvest HTB-19/HTB-20 cancer cells in order to continue genistein experiments followed by analysis of the presence or absence of acetylated Due to the shortcomings of previous treatment experiments there will be an i effort on proper technique during potential steps where protein or cells ma including the before mentioned PBS wash/centrifugation and overestimation o culture. Correcting these inefficiencies in our technique is a main priority i moving forward. Acknowledgements: Special thanks to Yutong Kang, Madeleine Hornick References: 1. Majid et al. (2008) Cancer Res. 68 (8), 2736-2744 2. Majid et al. (2009) Carcinogenesis. 30(4), 662–670 3. Donnelly et al. (2004) Am J Physiol Lung Cell Mol Physiol. 287, L774–L783 4. Tang et al. (2006) Mol Cancer Ther. 5(8), 2034-2042 5. Bandele,O and Osheroff,N. (2008) Biochemistry. 47, 11900–11908 6. Adlercreutz , H.(1995) Environmental Health Perspectives. 103 (7), 103-113 7. Ganesan et al. (2009) Current Cancer Drug Targets. 9, 963-981 8. Mense et al. (2008) Environmental Health Perspectives. 114 (4), 426-433 9. Dalvai, M and Bystricky, K. (2010) J Mammary Gland Biol Neoplasia. 15, 19–33 10. Rajah et al. (2009) Pharmacology .84, 68–73 11. Satih et al. (2009) Journal of Molecular Signaling. 4 (3), 42-56 12. Singh,R and Agarwal,R. (2006) Current Drug Targets. 7, 345-354 13.Srivastava et al. (2009) Mutation Research. 681, 180-188 14. Majid et al. (2010) Cancer. 116, 66-76