Plant Genetics, Breeding and Biotechnology (PLSC 452/552)
Lecture 18, Chapter 11
Analysis of transgenic plants part I
Mat Halter
3/27/12
Plant Genetics, Breeding and Biotechnology (PLSC
452/552),
University of Tennessee
Lecture 19, Chapter 11
Analysis of transgenic plants part II
Neal Stewart
Hin d III (11012)
Sph I (11010)
Pst I (11004)
Sal I (10994)
Xba I (10988)
Bam H I (10982)
Sma I (10979)
Kpn I (10977)
Sac I (10971)
Eco R I (10961) lacZ alpha
CaMV 35S promoter
Gus first exon
Nco I (1)
Bgl II (8)
Catalase intron
Bst XI (10718)
CaMV35S promoter
Xho I (9931)
Bgl II (9918)
Nco I (9903) kanamycin (R)
Sph I (9373)
Xho I (9053)
CaMV35S polyA
T-Border (left) pCAMBIA2201
11773 bp
Gus second exon
Histidine tag
Nhe I (2014)
Pml I (2037)
Bst EII (2050) Nos poly-A
T-Border (right)
Sph I (2455) chloramphenical (R) pVS1 sta pBR322 ori pBR322 bom
Nhe I (5458) pVS1 rep
Transformation is a relatively rare event
.
• Therefore selection has been needed.
– NPTII
– Bar
• Recently, easily scorable and non-invasive markers.
Figure 9.3
Sometimes “escapes” occur– for kanamycin resistance markers tissue is red—very stressed
Stable integration of transgene
• Transgene is permanently integrated into the genome of the host plant.
• Transmitted to progeny (T n plants) in
Mendelian fashion
• Need convincing proof of stable integration
• Multiple assays are possible—but most researchers are best convinced by Southern blot data.
Fluorescent Proteins
http://en.wikipedia.org/wiki/File:FPbeachTsien.jpg
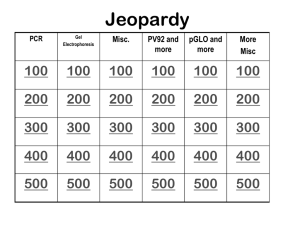
PCR analysis by gel electrophoresis
Ladder
1500 bp
1000 bp
Sample
+
750 bp
500 bp
PCR and False Positives
Genomic DNA
Transgenic plant produced from
Agrobacterium-mediated transformation
• In T
0 plants, Agrobacterium left over from the initial transformation is still present in all tissues.
• Contamination of the genomic DNA with the initial transformation vector that is still present in the agrobacterium can produce a PCR band.
Southern Blot
• Southern blotting confirms the presence of the gene of interest in the genomic DNA of the target plant and avoids the pitfalls of potential false positives.
• Steps
– Genomic DNA isolation
– Restriction enzyme digestion of genomic DNA
– Running digested DNA on agarose gel to separate fragmented DNA by size.
– Transfer of separated DNA to nylon membrane
– Hybridization with radioactive DNA probe
Digested Genomic
• Essentially, every known restriction enzyme will have cut sites in a plant genome.
• How can enzyme selection be used to detect copies of an inserted transgene?
EcoRI Site
DNA Probe
LB RB
• Single cutting enzymes can be designed into the T-DNA before transformation that will enable proper digestion of the genome as well as a single cut within the T-
DNA.
Why is a single cut within the T-DNA necessary?
EcoRI Site
LB
EcoRI Site
RB LB
If there is no EcoRI site within the tDNA, after digestion with
EcoRI these two insertion sites will be indistinguishable from one another after electrophoresis and probing.
Cutting within the T-DNA is necessary to distinguish each and every insertion event. This is VERY important.
Restriction digest and gel electrophoresis http://www.ndpteachers.org/perit/Electrophoresis%20%5B2%5D.gif
Southern blot—DNA transfer to nylon
www.gbiosciences.com/Southern-Blot-desc.aspx
Northern blot analysis
• Gives relative amount of gene expression-at the transcript level.
• Isolate mRNA be a lot and of good quality (not degraded)
• Separate transcripts on a gel
• Transfer to nylon filter
• Probe filter with DNA of interest (transgene) http://www.youtube.com/watch?v=KfHZFyADnNg
No digestion necessary… why is this?
RNA loading controls are necessary to ensure an equal amount of RNA is loaded in each well.
Northern Blot
Figure 11.9
Northern blot example
What is missing in this experiment?
Western blot
• Also to measure gene expression—at the protein level.
• Extract proteins
• Separate proteins on a vertical gel
• Transfer to a membrane using an electrotransfer system
• Probe with antibodies.
• Stain for antibodies
Western blots and ELISAs often use amplification of signal via antibodies http://probes.invitrogen.com/handbook/images/g001474.gif
Figure 11.11
Western blot example
What is missing in this experiment?
Real-time PCR or Quantitative PCR
• Real-time PCR uses fluorescence as an output for DNA amplification in real-time.
• The amount of starting template DNA (or cDNA for RNA measurement (real-time RT-
PCR) is correlated with the Ct number.
• More DNA = lower Ct; Ct is the cycle number when a threshold amount of DNA is produced during the PCR experiment.
http://www.rt-pcr.com/ http://www.youtube.com/watch?v=QVeVIM1yRMU
Advantages of qRT-PCR over RT-PCR?
• Is my plant transgenic?
– Survives selection
– Reporter gene expression
– Progeny analysis
– PCR
– Southern blot analysis
Summary
• Is my plant expressing the transgene?
– Northern blot analysis
– Western blot analysis
– ELISA
– RT-PCR
– Real-time RT PCR