- IREP - International Islamic University Malaysia
advertisement
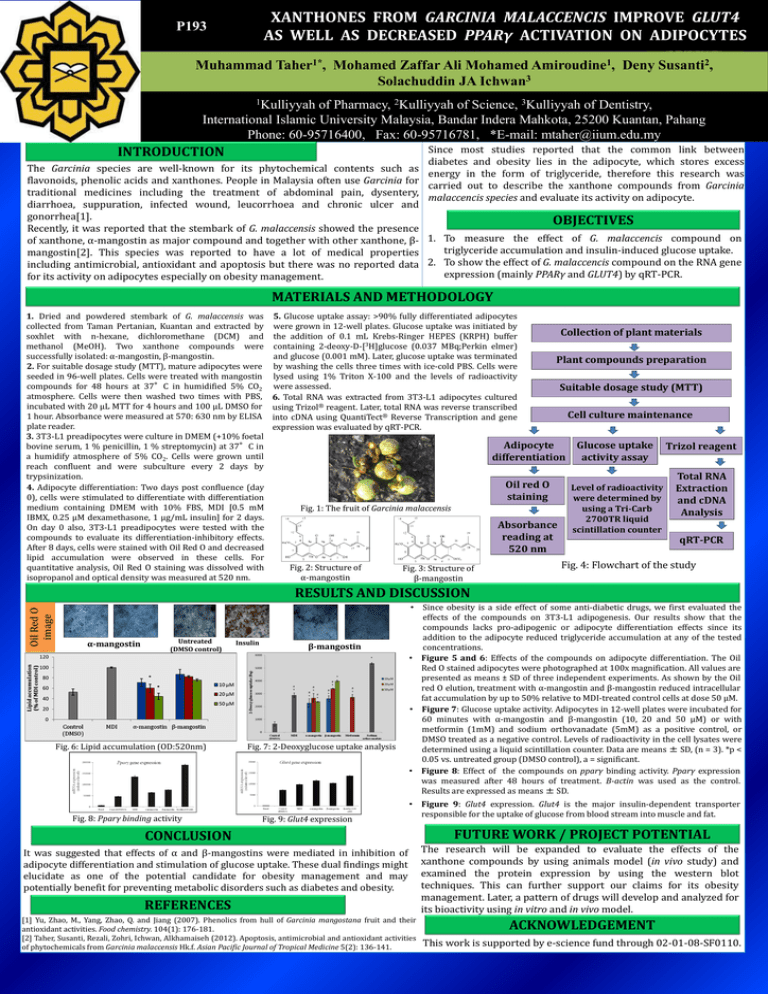
XANTHONES FROM GARCINIA MALACCENCIS IMPROVE GLUT4 AS WELL AS DECREASED PPARγ ACTIVATION ON ADIPOCYTES P193 Muhammad Taher1*, Mohamed Zaffar Ali Mohamed Amiroudine1, Deny Susanti2, Solachuddin JA Ichwan3 1Kulliyyah of Pharmacy, 2Kulliyyah of Science, 3Kulliyyah of Dentistry, International Islamic University Malaysia, Bandar Indera Mahkota, 25200 Kuantan, Pahang Phone: 60-95716400, Fax: 60-95716781, *E-mail: mtaher@iium.edu.my Since most studies reported that the common link between diabetes and obesity lies in the adipocyte, which stores excess The Garcinia species are well-known for its phytochemical contents such as energy in the form of triglyceride, therefore this research was flavonoids, phenolic acids and xanthones. People in Malaysia often use Garcinia for carried out to describe the xanthone compounds from Garcinia traditional medicines including the treatment of abdominal pain, dysentery, malaccencis species and evaluate its activity on adipocyte. diarrhoea, suppuration, infected wound, leucorrhoea and chronic ulcer and gonorrhea[1]. OBJECTIVES Recently, it was reported that the stembark of G. malaccensis showed the presence of xanthone, α-mangostin as major compound and together with other xanthone, β- 1. To measure the effect of G. malaccencis compound on triglyceride accumulation and insulin-induced glucose uptake. mangostin[2]. This species was reported to have a lot of medical properties including antimicrobial, antioxidant and apoptosis but there was no reported data 2. To show the effect of G. malaccencis compound on the RNA gene expression (mainly PPARγ and GLUT4) by qRT-PCR. for its activity on adipocytes especially on obesity management. INTRODUCTION MATERIALS AND METHODOLOGY 1. Dried and powdered stembark of G. malaccensis was collected from Taman Pertanian, Kuantan and extracted by soxhlet with n-hexane, dichloromethane (DCM) and methanol (MeOH). Two xanthone compounds were successfully isolated: α-mangostin, β-mangostin. 2. For suitable dosage study (MTT), mature adipocytes were seeded in 96-well plates. Cells were treated with mangostin compounds for 48 hours at 37°C in humidified 5% CO2 atmosphere. Cells were then washed two times with PBS, incubated with 20 µL MTT for 4 hours and 100 µL DMSO for 1 hour. Absorbance were measured at 570: 630 nm by ELISA plate reader. 3. 3T3-L1 preadipocytes were culture in DMEM (+10% foetal bovine serum, 1 % penicillin, 1 % streptomycin) at 37°C in a humidify atmosphere of 5% CO2. Cells were grown until reach confluent and were subculture every 2 days by trypsinization. 4. Adipocyte differentiation: Two days post confluence (day 0), cells were stimulated to differentiate with differentiation medium containing DMEM with 10% FBS, MDI [0.5 mM IBMX, 0.25 µM dexamethasone, 1 µg/mL insulin] for 2 days. On day 0 also, 3T3-L1 preadipocytes were tested with the compounds to evaluate its differentiation-inhibitory effects. After 8 days, cells were stained with Oil Red O and decreased lipid accumulation were observed in these cells. For quantitative analysis, Oil Red O staining was dissolved with isopropanol and optical density was measured at 520 nm. 5. Glucose uptake assay: >90% fully differentiated adipocytes were grown in 12-well plates. Glucose uptake was initiated by the addition of 0.1 mL Krebs-Ringer HEPES (KRPH) buffer containing 2-deoxy-D-[3H]glucose (0.037 MBq;Perkin elmer) and glucose (0.001 mM). Later, glucose uptake was terminated by washing the cells three times with ice-cold PBS. Cells were lysed using 1% Triton X-100 and the levels of radioactivity were assessed. 6. Total RNA was extracted from 3T3-L1 adipocytes cultured using Trizol® reagent. Later, total RNA was reverse transcribed into cDNA using QuantiTect® Reverse Transcription and gene expression was evaluated by qRT-PCR. Collection of plant materials Plant compounds preparation Suitable dosage study (MTT) Cell culture maintenance Adipocyte differentiation Oil red O staining Fig. 1: The fruit of Garcinia malaccensis Absorbance reading at 520 nm Fig. 2: Structure of α-mangostin Fig. 3: Structure of β-mangostin Glucose uptake activity assay Level of radioactivity were determined by using a Tri-Carb 2700TR liquid scintillation counter Trizol reagent Total RNA Extraction and cDNA Analysis qRT-PCR Fig. 4: Flowchart of the study Oil Red O image RESULTS AND DISCUSSION α-mangostin Untreated (DMSO control) Fig. 6: Lipid accumulation (OD:520nm) Fig. 8: Pparγ binding activity Insulin β-mangostin Fig. 7: 2-Deoxyglucose uptake analysis Fig. 9: Glut4 expression • Since obesity is a side effect of some anti-diabetic drugs, we first evaluated the effects of the compounds on 3T3-L1 adipogenesis. Our results show that the compounds lacks pro-adipogenic or adipocyte differentiation effects since its addition to the adipocyte reduced triglyceride accumulation at any of the tested concentrations. • Figure 5 and 6: Effects of the compounds on adipocyte differentiation. The Oil Red O stained adipocytes were photographed at 100x magnification. All values are presented as means ± SD of three independent experiments. As shown by the Oil red O elution, treatment with α-mangostin and β-mangostin reduced intracellular fat accumulation by up to 50% relative to MDI-treated control cells at dose 50 µM. • Figure 7: Glucose uptake activity. Adipocytes in 12-well plates were incubated for 60 minutes with α-mangostin and β-mangostin (10, 20 and 50 µM) or with metformin (1mM) and sodium orthovanadate (5mM) as a positive control, or DMSO treated as a negative control. Levels of radioactivity in the cell lysates were determined using a liquid scintillation counter. Data are means ± SD, (n = 3). *p < 0.05 vs. untreated group (DMSO control), a = significant. • Figure 8: Effect of the compounds on pparγ binding activity. Pparγ expression was measured after 48 hours of treatment. B-actin was used as the control. Results are expressed as means ± SD. • Figure 9: Glut4 expression. Glut4 is the major insulin-dependent transporter responsible for the uptake of glucose from blood stream into muscle and fat. CONCLUSION It was suggested that effects of α and β-mangostins were mediated in inhibition of adipocyte differentiation and stimulation of glucose uptake. These dual findings might elucidate as one of the potential candidate for obesity management and may potentially benefit for preventing metabolic disorders such as diabetes and obesity. REFERENCES [1] Yu, Zhao, M., Yang, Zhao, Q. and Jiang (2007). Phenolics from hull of Garcinia mangostana fruit and their antioxidant activities. Food chemistry. 104(1): 176-181. [2] Taher, Susanti, Rezali, Zohri, Ichwan, Alkhamaiseh (2012). Apoptosis, antimicrobial and antioxidant activities of phytochemicals from Garcinia malaccensis Hk.f. Asian Pacific Journal of Tropical Medicine 5(2): 136-141. FUTURE WORK / PROJECT POTENTIAL The research will be expanded to evaluate the effects of the xanthone compounds by using animals model (in vivo study) and examined the protein expression by using the western blot techniques. This can further support our claims for its obesity management. Later, a pattern of drugs will develop and analyzed for its bioactivity using in vitro and in vivo model. ACKNOWLEDGEMENT This work is supported by e-science fund through 02-01-08-SF0110.











