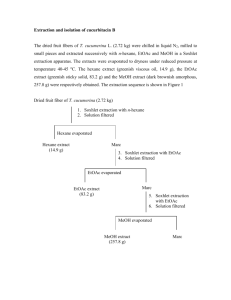
ISOLATION AND STRUCTURE IDENTIFICATION OF BIOACTIVE
advertisement

ISOLATION AND STRUCTURE IDENTIFICATION OF BIOACTIVE COMPOUNDS FROM LEAVES AND TWIGS OF Garcinia succifolia Kurz (GUTTIFERAE) Rangsinee Hamkool1,*, Pawinee Piyachaturawat2, Surawat Jariyawat2, Kanoknetr Suksen2, Darunee Soorukram1, Manat Pohmakot1 , Vichai Reutrakul1, Patoomratana Tuchinda1 1Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahidol University, Bangkok 10400, Thailand. 2 Department of Physiology, Faculty of Science, Mahidol University, Bangkok 10400, Thailand. *e-mail: rhamkool@gmail.com, #e-mail: patoomratana.tuc@mahidol.ac.th. Abstract Plants of the genus Garcinia were found to possess a wide range of biological activities, such as antimicrobial, cytotoxic, antitumor, antimalarial, antifungal and antioxidant activities. Garcinia succifolia Kurz (Guttiferae) is an endemic plant, found in Northern Thailand (Chiang Mai Province). Therefore, we are interested in the isolation and identification of compounds from a methanol extract of leaves and twigs. The crude extracts, fractions and isolated pure compounds were evaluated for their cytotoxic activities against a panel of mammalian cancer cell lines. Keywords: Garcinia succifolia, xanthones, benzophenone, triterpenoids Introduction Garcinia is the most numerous genus with about 400 species widely distributed in Tropical Asia, Africa, New Caledonia and Polynesia, being widespread throughout the tropical and subtropical regions of the world.1 In Thailand, there are twenty six species of Garcinia having been reported, namely, G. acuminata, G. atroviridis, G. bancana, G. cambogia, G. costata, G. cowa, G. dulcis, G. forbesii, G. fusca, G. garcilis, G. hanburyi, G. hombroniana, G. lanessanii, G. mackeaniana, G. mangostana, G. merguensis, G. nervosa, G. nigrolineata, G. parvifolia, G. rostrata, G. schomburgkiana, G. speciosa, G. succifolia, G. thorelii, G. vilersianaand, and G. xanthochymus.2 The studies of several Garcinia species indicated the presence of a variety of chemical constituents. The genus Garcinia (Guttiferae family) is known as a rich source of polyisoprenylated, benzophenones, xanthones, biflavonoids, triterpenoids and biphenyl derivatives. Several Garcinia species were found to possess a wide range of biological activities such as antimicrobial3, cytotoxic5,7, antitumor9, antimalarial8, antifungal4 and antioxidant5,6activities. Methodology The air-dried and finely ground powder from leaves and twigs of Garcinia succifolia were extracted with methanol at room temperature, followed by filtration. The filtrates were combined and evaporated to dryness under reduced pressure to give a crude methanol extract. The extract was evaluated for the cytotoxic activity. The crude methanol extract was dissolved with EtOAc:MeOH (1:1) at room temperature, followed by MeOH. The crude EtOAc:MeOH (1:1) was first separated by column chromatography. Further purification by repeated column chromatography and crystallization afforded macluraxanthone (1), friedelin (2), caloxanthone C (3), lichexanthone (4), stigmasterol (5) and dichromene II (6). Results and Discussion The bioactive MeOH:EtOAc (1:1) soluble fraction (see Table 1), obtained from a crude MeOH extract of leaves and twigs of G. succifolia, was separated by column chromatography and recrystallization to yield six known compounds 1-6. Table 1. Cytotoxic activities of the extract, fractions and pure isolated compounds Code a Cell line P-388 KB HT 29 MCF-7 A 549 ASK Hek 293 Ellipticine 0.10 0.53 0.49 0.45 0.45 0.66 0.45 MeOH extract 10.6 16.29 >20 11.93 >20 >20 17.17 MeOH:EtOAc (1:1) 10.41 16.45 >20 11.88 >20 >20 11.28 MeOH >20 >20 >20 >20 >20 >20 >20 Residue >20 >20 >20 >20 >20 >20 >20 F1 >20 >20 >20 >20 >20 >20 >20 F2 8.18 >20 >20 >20 >20 >20 >20 F3 <4 11.46 12.78 9.29 13.38 18.53 11.23 F4 <4 <4 10.28 <4 <4 10.72 <4 F5 3.95 16.04 >20 13.24 >20 >20 15.91 F6 14.87 >20 >20 >20 >20 >20 >20 F7 >20 >20 >20 >20 >20 >20 >20 Ellipticine 0.43 0.54 0.56 0.56 0.51 0.47 0.57 1 1.45 2.16 7.8 1.26 2.22 1.8 5.25 2 >20 >20 >20 >20 >20 >20 >20 3 >20 >20 >20 >20 >20 >20 >20 Results are expressed as ED50 (µg/mL): ED50 < 4 µg/mL is considered active for pure compound. *P-388 = Murine lymphocytic leukemia, KB = Human nasopharyngeal carcinoma, HT29 = Human colorectal adenocarcinoma, MCF-7 = Human breast cancer, A549 = Human lung carcinoma, ASK = Cell line from rat glioma, Hek 293= Non-cancerous human embryonic kidney cell line. Conclusion Investigation of the MeOH extract of leaves and twigs of Garcinia succifolia has led to the isolation of macluraxanthone (1), friedelin (2), caloxanthone C (3), lichexanthone (4), stigmasterol (5) and dichromene II (6). Fig1. The structures of compounds 1-6 isolated from leaves and twigs of Garcinia succifolia Kurz. References: 1. 2. 3. 4. 5. 6. 7. 8. 9. Whitmore TC. Guttiferae. Free flora of Malaya.Vol. 2, Forest Research Institute, Kepong. 1973;2:162-165. Smitinand T. Thai plant names; Pra Cha Chon Co., Ltd.: Bangkok, 2001, Revised Edition by The Forest Herbarium Royal Forest Department, 246-248. Pattalung PN, Wiriyachitra P, Ongsakul M. The antimicrobial activities of rubraxanthone isolated from Garcinia parvifolia (Miq) Miq. J Sci Soc. Thailand. 1988;14:67-71. Diserens IS, Marston A, Hamburger M, Rogers C, Hostettmann K. Novel prenylated xanthones from Garcinia gerrardii Harvey. Helv Chim Acta. 1989;72:1001-1007. Lin LJ, Lin ZL, Pezzuto JM, Coedell GA. Isogambogic acid and isomorellinol from Garcinia hanburyi. Magn Reson Chem. 1993;31:340-347. Minami H, Kuwayama A, Yoshizawa T, Fukuyama Y. Novel prenylated xanthones with antioxidant property from the wood of Garcinia subelliptica. Chem Pharm Bull. 1996;44(11):2103-2106. Asano J, Chiba K, Tada M, Yoshii T. Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry. 1996; 41(30):815-820. Iio C, Miyamoto Y, Nakayama M, Kawai Y, Rao K , Furukawa H. A novel depsidone and some new xanthones from Garcinia species. Chem Pharm Bull. 1997;45(9):1403-1413. Nilar, Harrrison LJ. Xanthones from the heartwood of Garcinia mangostana. Phytochemistry. 2002;60(5): 541-548. Acknowledgement: This research project is supported by Mahidol University, the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative and Center for Innovation in Chemistry (PERCH-CIC).