Team E
advertisement

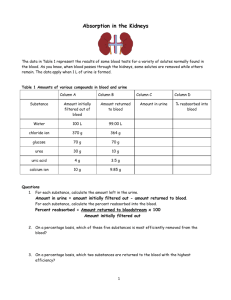
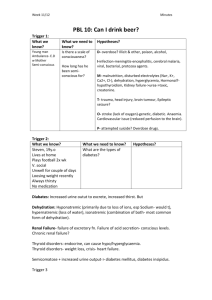
Alan Dorsinville, Reading High School Natalie Gibbs, Reading High School Introduction The Problem and our solution Background Information The purpose of μPAD’s Materials Procedure Science Concepts Results Team F(Floral Experiment) Conclusion/Discussion Further Research Acknowledgements The cost of health care is an issue in America Testing requires Time Money Insurance Technical Experience Etc All of which is inconvenient Developing Countries Micro fluidic paper-based assay devices (μPAD) A paper diagnostic test Paper-based devices are Inexpensive Quick Easy to use Require a small volume of liquid Lack the use of advanced equipment Effective and accurate Your body has many substances including proteins and glucose Glucose Concentration Glucose provides energy 0-0.8mM for your body an all of your movements Above 0.8mM Disease Normal Impaired kidney and/or diabetes Proteins are important for growth, tissue repair, and many other bodily functions Our purpose is to show we can quantify diagnostic results using a cheap paper device Our chips are designed to detect glucose and protein in our substances Intermolecular forces Capillary action Allows us to direct small amounts of liquid to testing wells CleWin Chromatography paper( Whatman) Printer Scales, beakers, pipettes Various chemicals Infrared Gun Hot Plate Scanner Adobe Photoshop Part One: Planning CleWin is a computer program made to design our chips Step 1: Printing The pattern is outlined with wax when printed Step 2: Place the chips on a hot plate at 150°C Allows wax to seep through This project requires making both a protein and glucose reagent A chemical reagent is a substance used in a chemical reaction to detect, measure, examine, or produce other substances Buffer (pH=6.0) 0.2 M NaH2PO4 0.2 M Na2HPO4 0.3 M Trehalose 0.6 M KI 30 units/mL HRP 120 units/mL GO Glucose + Glucose oxidase Gluconic acid + Hydrogen peroxide (H2O2) H2O2 HR Peroxidase I- H2O + ½ O2 ½ I2 Brown color Part 1: 0.25 M Citric acid (pH 1.8 buffer) 184 μL H2O, 16 μL EtOH Part 2: 9 mM TBPB 10 μL H2O, 190 μL EtOH Protein Mechanism TBPB + protein = Blue color Apply 0.2 μL of reagents using a micropipette. Must wait ten minutes Part Five: Test One We made a new design for our second chip on CleWin, printed them, and reapplied the chemical reagents, etc. The chips are a urine analysis test so we made an artificial urine sample We made several concentrations glucose and proteins to test Sol'n 1 Glucose BSA Urine 25 25 0 2 18.75 20.83 10.42 3 12.5 16.67 20.83 4 9.375 12.5 28.13 5 6.25 8.33 35.42 6 5 4.17 40.83 7 3.75 3.125 43.13 8 2.5 2.08 45.42 9 1.25 0.833 47.92 10 0.63 0.42 48.96 11 0 0 50 We performed eight tests for each of the eleven different concentrations of glucose and protein After 30 minutes, the chips were scanned into the computer Protein (BSA) 240 y = 0.694x + 184.6 R² = 0.984 230 220 Mean Intensity 210 200 190 180 170 160 150 0 10 20 30 40 BSA Concentration (μM) 50 60 70 Glucose 150 Mean Intensity 145 140 135 130 125 0 5 10 15 Glucose Concentration mM 20 25 Team F’s research is focused on the relationship between pollinators and nectar Different Nectar Concentrations 170 160 150 Intensity 140 130 120 110 100 Partridge Pea Sunflower Thistle Thistle (2nd) Nectar Type Mallow Blank We made adjustments to our second chip to create a more effective test We were not able to quantify, but still proved the chips had the potential to quantify different detectable substances We were able to rank the glucose concentration of Team F’s nectar solutions The μPAD’s serve the same purpose as other urine tests Scientists are trying to find ways of detecting more diseases with the paper-based analytical device We would like to thank: Dr. Scott Phillips SeeCos Faculty Chris Daly Ms. Jody Markley Mr. Derek James Ms. Jean Marie Donnelly UBMS Staff