Slides for Part I & II
advertisement

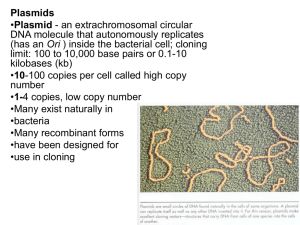
DIY Molecular Cloning I: DNA Overview, Extracting DNA, Finding genetic sequences, Designing a cloning strategy C. Rouskey Jan.2014 Intros ...name and background, what you hope to learn... Me GETit Outline DNA Structure and Function/DNA Extraction Sequences on the Web/Determining your GOI Choosing a plasmid Components (OriC, Promoter, Reporters, GOI) Designing a cloning Strategy Cloning System? Eukaryotic or Prokaryotic Cloning GETit Cloning Work DNA DNA Basics Deoxyribonucleic Acid Stores information Made up of nucleotides Adenine (p), Cytosine (py), Thymine (py), Guanine (p) with a deoxyribose backbone; RNA T = U(racil) These nucleotides bind in complement to each other via hydrogen bonds (G:::C; A::T(U)) Hydrogen bonds are fairly weak when taken on their own, but the cumulative forces of these bonds make DNA stable 5' and 3' ends Two strands running in the opposite direction Sequences are reverse complement What kind of DNA? Prokaryotic or Eukaryotic Genomic Plasmid DNA Replication Where does it occur? How does it occur (overview)? Major enzyme? What are the results? DNA Transcription Where does it occur? How does it occur? Promoter, ATG What are the results? Start and Stop Codons DNA Translation Where does it occur? How does it occur? Ribosomes/tRNA, Start/Stop Codons What are the Results? PROTEINS Overview: Cloning with REs What is molecular cloning? tools used to create recombinant DNA molecules General steps: Amplify (via PCR) gene with RE sequences present Digest PCR product and plasmid/Purify Ligate PCR product into Plasmid Transform E. coli – look at expression or purify plasmid Molecular Biologist Toolkit Genetic Databases – resource for sequences Online analysis tools – primer design, restriction assessment DNA/RNA – the genetic starting material Plasmids – functional holding place for gene Primers – directs DNA amplification, targets specific genes Enzymes – used in the amplification cutting and ligation of DNA. Host Strains – amplify and express your GOI Online Resources Gene search: http://www.pubmed.com Restriction analysis: http://tools.neb.com/NEBcutter2 Primer assessment: http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/ Composite program: Serial Cloner Plasmids Autonomously reproducing circular DNA Origin of replication determines copy number (OriC) Careful not to create a bio-burden on your cells Plasmids Promoter - drives the transcription of GOI Multiple cloning Site – Space to pop in GOI downstream of promoter Reporters – let you assess if GOI is being expressed Purification Tags – helpful in downstream purification of proteins (useful tool for functional protein studies Primers Strands of DNA that serve as the starting point for DNA synthesis Host Strains E. coli is the basic cloning host for growing plasmid and expressing prokaryotic proteins. E. coli can be used to propagate plasmid containing eukaryotic genes but expression is not really useful (post-translational modifications present in Euks) Competent cells! E. cloni 10G (super easy), Can make your own competent cells After plasmid is made, pop it into E. coli GETit Specific Cloning (example) Quick GETit background Bacteriophage therapy Lytic vs. Lysogenic Phage (for more info visit http://getitproject.org/getitproject-meetup-112313/ Cloning (GETit) 1. Find the genetic sequences of interest http://www.pubmed.com a. are there phage in the N. gonorrhoeae genome? Cloning Strategy Find a plasmid that you can clone into: What are you trying to accomplish? What kind of gene is it? What do you want to do with the genetic material? Order plasmid from addgene (http://addgene.com) We want a moderate copy number, promoter, antibiotic resistance gene GOI: phage tail protein I (187aa) 5'TCACACTGCACAGCCCCGCTGCCGACATCGAATGCGGCAACGGCGAATAC ATCCGAATCACGTCCACGCTCGAGCGCGAATAAATGAACAGCACCGTCCCC GCGAACAACAGCCCCCTGCAACACGCACTGGCAAAGCTGACAGAACGCGA AACTGCCGCCGTCTCCCGCCAACTCGACCCCGCCCGATGCGACCCCGGAT TTTTACCCTTTCACGCCTTCGCAAGAAGCATCGGCACGGAAGAGGGCTGG GACTTTGCCGAAACCGACGAAGCCCGCCGCAACCTCATCGCAGGCTTTGC CGAAATCCACGCCCGAAAAGGCACGCCGTACGCCATCCGCGCCCTCTTCC CCATCTTGCGGCTGGGCGAAATCCAAATTATCGAACGCGACGGCGAGTTCA AGTGGGACGGCTCGGTCTTGTTCGACGGCAGCCGCACATTCGGCAGGCG CGAGGGTGACTGGGCGGAATACCGCATTGTCTTAACGCGCCCCGTCAGCA TCCGCCAAACCGCCCGCATCCGCGCCATGTTGGCGGAAATCGCCCCCTTG CGGTGCGAACTTACCGCGCTCGACTACCGCAACCATCCCCACCGCTGGAA CGGCAAAATCCGCTTTAACGGCGAATACGGTTTCGGCACGACATAACGCGC CCCCGAAAATCAACAAACAAAAGGAAGCCCCAAAATGGCAAACGCAACCGA ACAAAACCAATTCGACCAAGCCGTCCGCC-3' Enzymes DNA Polymerase (used to amplify template) Restriction endonucleases cut at specific sites Ligase Deciding where to clone? 1. I like BamHI and EcoRV (can I cut them together?) http://66.155.211.155/nebecomm/DoubleDigestCalculator.asp BamHI 2. Analyze Gene Sequence - Are there restriction sites internally? http://tools.neb.com/NEBcutter2/! EcoRV Primer Design 1. Determine Primer sequence for PCR... 5' end... Sequence: 5' TCACACTGCACAGCCCCGCTGCCGACATCGAATGCGGCAA Primer: 5' - CCGCTGCCGGATCCGAATG 3' end... AAAACCAATTCGACCAAGCCGTCCGCC – 3' Sequence: 5' - 3' - TTTTGGTTAAGCTGGTTCGGCAGGCGG – 5' Primer: 3' - TTTTGGTTGATATCGTTCGGCAGGCGG – 5' 5' - GGCGGACGGCTTGCTATAGTTGGTTTT – 3' DNA Sequence 5' – TCACACTGCACAGCCCCGCTGCCGACATCGAATG -3' Primer 5' - Complement 3' - AGTGTGA.... Reverse Complement 5' – CATTCGAT.... CCGCTGCCGGATCCGAATG Now...PCR Restriction Digest Set up a restriction digest reaction using BamHI and EcoRV Digest Plasmid and PCR product Analyze digest on gel Ligate and Transform! 18 hours later. . . Downstream Applications Sequencing (confirm protein) Protein Expression/Analysis via PAGE Protein Purification Experiment 1. Find the genetic sequence http://banana-genome.cirad.fr/musa 2. Design primers 3. Extract DNA from Musa acuminata...(Protocol) 4. PCR