PowerPoint 簡報
advertisement
Comparison of Proportions
Part II
Instructor: 李奕慧
yihwei@mail.tcu.edu.tw
1
Lecture Overview
Study designs in epidemiology
Measures of study effect for 2
categorical variables
1. Risk difference
2. Relative risk
3. Odds ratio
McNemar’s test for Matched-pair
study
2
Epidemiologic Study Design
Analytical studies
Intervention
studies
Clinical trials
Observational studies
Cross-sectional studies
Cohort studies
Case-control studies
3
Measures of Study Effect
For cohort and cross-sectional studies only
RD p1 p2
risk difference
RR p1 / p2
risk ratio/relative risk
For any type of study
p1 / q1
OR
p2 / q2
odds ratio
4
Risk difference (RD, p1-p2)
戴安全帽
頭部
合計
受傷 是
否
是
12
62
74
否
88
38 126
合計 100 100 200
第一組:意外發生時機車騎士沒有戴安全帽
p1:第一組母體比例
第一組樣本
n1=100, pˆ 1 (頭部受傷比例)=0.62
第二組:意外發生時機車騎士有戴安全帽
p2: 第二組母體比例
第二組樣本:n2=100,
pˆ 2 =0.12
5
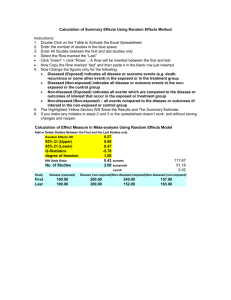
建構 p1-p2 的信賴區間(confidence interval, CI)
( pˆ 1 pˆ 2 ) Z / 2
pˆ 1 (1 pˆ 1 ) pˆ 2 (1 pˆ 2 )
n1
n2
where
pˆ 1 pˆ 2 0 . 5 ,
Z 0 .025 1 . 96
0.62x0.38 0.12x0.88
0.0584
100
100
0.5 1.96x0.0584 = (0.386, 0.614)
沒戴安全帽頭部受傷的機率,
較有戴安全帽者高出
39%~61%,戴安全帽的機
車騎士,在車禍發生時,可
以減少39%~61%的頭部受
傷的機會。
6
檢定 H0:p1-p2=0 versus Ha:p1-p20
Z
ˆ1 p
ˆ 2 ) ( p1 p2 )
(p
ˆ1 (1 p
ˆ1 )
p
n1
ˆ 2 (1 p
ˆ2 )
p
n2
Z = 0.5/0.058 =8.56, P-value < 0.001
Reject H0, there are significant differences in the population
proportions between the two groups.
7
2 sample
proportion
test.xls
8
Example for Clinical trial and Risk Difference
BMJ 2006;333;11939
比較Intervention group與Control group病人
住院3天後的死亡率(death after day 3)
每一個病人皆觀察其死亡(=1)或存活(=0)情形
Intervention group:5/132 (5%)的人住院3天後死
亡
Control group: 8/133 (8%)的人住院3天後死亡
兩組住院3天後死亡率的差異(control-intervention)
為 2% (point estimate), 95%CI:(-3% to 8%),表
示兩組母體死亡率差異的範圍介於-3%~8%,
Intervention不會影響病人的死亡率。
10
Cohort study / Clinical trial
Disease +
Exposed
(Intervention)
Disease -
Study population
Disease +
Non-exposed
(Control)
Disease -
11
Cohort Data 2 x 2 table
ill
not ill
Exposed
a
b
Unexposed
c
d
Incidence in exposed (p1)
= a/(a+b)
Incidence in non-exposed(p2) = c/(c+d)
12
Effect measures in cohort studies
Hypothesis
Is
the incidence among exposed higher than
among unexposed
Absolute measures
Risk
difference (RD)
Relative measures
Relative
P1 P2
risk/Risk Ratio (RR)
P1
P2
13
Foodborne Outbreak in a Wedding, Dublin
Ate
ham
Did not
eat ham
ill
not ill
Incidence
49
49
98
50%
4
6
10
40%
53
55
108
Risk difference
0.5 - 0.4 = 0.1 (10%)
Relative risk
0.5 / 0.4 = 1.25
14
RR與OR的意義
RR=1
RR>1
RR<1
ln(RR)=0
ln(RR)>0
ln(RR)<0
沒有相關
危險因子
保護因子
15
Relative risk
RR = 1.25
RR = 1.13
RR = 13
RR= 0.8
25 % increase in risk
13 % increase in risk
13 fold increase
20% of risk reduction
16
Example for Relative Risk
A Randomized Trial of Aspirin on the Risk of
Embolic Events in Patients With Infective
Endocarditis
JACC, 2003;42(5):775–80
17
aspirin * bleeding Crosstabulation
Bleeding ((果)
Yes(=1) No(=2)
Total
Count
17
42
59
Aspirin Yes(=1)
% within aspirin
28.8% 71.2% 100.0%
(因)
No(=2)
Count
8
47
55
% within aspirin
14.5% 85.5% 100.0%
Total
Count
25
89
114
% within aspirin
21.9% 78.1% 100.0%
Pearson Chi-Square
Continuity Correctionb
Likelihood Ratio
Fisher's Exact Test
Linear-by-Linear
Association
N of Valid Cases
Chi-Square Tests
Asymp. Sig. Exact Sig. (2- Exact Sig. (1(2-sided)
sided)
sided)
Value
df
a
3.385
1
.066
2.603
1
.107
3.455
1
.063
.074
.053
3.355
1
.067
114
a. 0 cells (.0%) have expected count less than 5. The minimum expected count is 12.06.
b. Computed only for a 2x2 table
Aspirin.sav
18
Risk Estimate
95% Confidence
Interval
Odds Ratio for aspirin (yes / no)
For cohort bleeding = yes
For cohort bleeding = no
N of Valid Cases
Value
2.378
Lower
.931
Upper
6.074
1.981
.930
4.218
.833
.685
1.013
114
OR = (17/42)/(8/47) = 2.38
RR = (17/59)/(8/55) =1.98
Aspirin.sav
19
Case-control (Retrospective)
Exposed
Cases
Non-exposed
Study Population
Exposed
Controls
Non-exposed
20
Odds ratio in Case-control and Cohort study
有子宮頸癌
CASE
150
無子宮頸癌
Control
50
Total
Nonsmoker
150
250
400
Total
300
300
600
Smoker
200
OR for smoking =(150/150)/(50/250) = 5
OR for cervical cancer = (150/50)/(150/250) = 5
不管是針對暴露因子,或疾病的odds ratio都相等,
odds ratio不會因為研究設計而有所改變。
21
Relative Risk and Odds ratio
P( Disease | exposed) {1 P( Disease | exposed)}
OR
P( Disease | un exposed) {1 P( Disease | un exposed)}
(cohortstudy)
P(exposed | Case) /{1 P( Disease | Case)}
P(exposed | Control) /{1 P(exposed | Control)}
(case controlstudy)
P( Disease | exposed)
RR
P( Disease | un exposed)
當疾病發生的機率很低時,OR RR
22
smoking * Cervical_cancer Crosstabulation
smoking
Total
Yes
(=1)
Count
% within Cervical_cancer
Cervical_cancer
Yes (=1) No(=2)
150
50
50.0% 16.7%
No
(=2)
Count
% within Cervical_cancer
150
250
50.0% 83.3%
Count
% within Cervical_cancer
Total
200
33.3%
400
66.7%
300
300
600
100.0%
100.0%
100.0%
Risk Estimate
Odds Ratio for smoking (yes / no)
Value
5.000
For cohort Cervical_cancer = yes
2.000
For cohort Cervical_cancer = no
.400
N of Valid Cases
600
95% Confidence Interval
Lower
Upper
3.424
7.302
1.722
.311
2.323
.515
Cervical
cancer2.sav
23
Matched Pair Study Design
Journal of Stroke and Cerebrovascular Diseases, 2005: pp 174-178
24
SBP control (<=140 mmHg) status before and
after stroke admission
Before
After
Control
Not
合計
Control
20 (67%)
10
30 (47%)
Not
14 (41%)
20
34
合計
34 (53%)
30
64
H0: Pbefore=Pafter
(中風前SBP控制的比例=中風後SBP控制的比例)
Ha: PbeforePafter
Stroke.sav
25
Paired categorical data, McNemar test
Before
After
Number of pts
Controlled
Controlled
20
Not controlled
Not controlled
20
Not Controlled
Controlled
14
Controlled
Not controlled
試問住院前後血壓控制情形有改善嗎?
10
26
Chi-Square Tests
Asymp. Sig. Exact Sig. Exact Sig.
Value df (2-sided) (2-sided) (1-sided)
Pearson Chi-Square
Fisher's Exact Test
McNemar Test
N of Valid Cases
4.158a
1
.041
.049
.036
.541c
64
a. 0 cells (.0%) have expected count less than 5. The minimum expected count is 14.06.
b. Computed only for a 2x2 table
c. Binomial distribution used.
應該使用McNemar檢定的結果,
而非一般的Chi-square檢定結果。
27
Thank you!
28