Immobilised Enzymes
advertisement

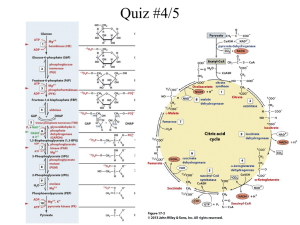
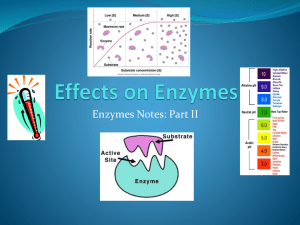
Chpt.’s 9 and 10 Metabolism • Is the sum of all the chemical reactions that take place in an organism – E.g. growth, movement, repair, response, reproduction • Cells need energy to maintain themselves • Metabolism is how cells obtain and use energy • Energy can either be released or absorbed – *Catabolic reactions breakdown complex molecules to simpler forms. Release energy. • E.g. respiration or digestion – *Anabolic reactions convert simple molecules into complex ones. Energy is consumed. • E.g. photosynthesis or polypeptide formation • Reactions take place in steps. These steps are carefully controlled by the cell. The most important controller of cellular reactions are enzymes Sources of Energy Solar Energy Cellular Energy Enzymes • A catalyst is a substance that speeds up a reaction without being used up • Enzymes are catalysts made of protein • Enzymes are biological catalysts that speed up reactions without being used up • Proteins are formed by joining a sequence of amino acids • The function of a protein is determined by » its amino acid sequence » its shape • Enzymes are folded into globular shapes • The 3D shape of an enzyme means it will fit and react only with a substance that has a matching shape • Anything that changes the shape of the enzyme will alter the efficiency of the enzyme » pH » temperature Enzyme action • Substrate – substance which an enzyme reacts with. • Product – substance(s) the enzyme forms. • Active Site – part of the enzyme that combines with the substrate. • Enzymes have a specific shape which determines their function. • Enzyme reactions are reversible. • This means any enzyme can be anabolic or catabolic • Naming Enzymes – add ase to name of their substrate Lipid (substrate) Lipase (enzyme) Inhibitors • chemicals that attach to an enzyme and destroy its shape i.e. denature enzyme Advantages – some insecticides, drugs and antibiotics act as inhibitors of enzymes not present in humans, therefore can act on bacteria etc. without harming humans e.g. Penicillin Disadvantages – many nerve gases are inhibitors that attach to enzymes involved in nerve impulse transmission e.g. cyanide – denatures enzyme involved in respiration. The Role of Enzymes • Catabolic and anabolic enzymes – see handout Factors affecting enzyme activity: • All enzymes work best under ideal conditions – optimal conditions – any change in these conditions will slow down the rate of the reaction by changing the enzymes effectiveness. • Will be investigating two of these conditions: - temperature - pH values Temperature – Human enzymes work best at 37oC – Plant enzymes work best at 20 – 30oC • At very low temps cells freeze and enzymes can not function • At very hot temps enzymes lose their shape and can not function (denatured above 50oC) TEMPERATURE AND RATE OF ENZYME ACTION pH pH scale 0 to 14 Enzymes work in a very narrow pH range Outside range activity falls rapidly Optimal pH for most enzymes is pH 7 0 7 acid neutral 14 base Temp and pH effect on activity Immobilised Enzymes Bio-processing: is the use of enzyme controlled reactions to produce a product Traditionally micro-organisms such as bacteria and yeast were used but since the 1900’s and especially since the 1950’s enzymes are being used. Bio-processing can be used to produce a vast range of products such as cheeses, beer, antibiotics, vaccines, methane gas, food flavours, vitamins and perfumes Immobilised Enzymes • If enzymes are used freely dissolved in a vessel it can be very wasteful as they are lost at the end of the process. • To prevent this problem enzymes are often immobilised or fixed. • This means they are attached to each other or an inert substance and can be used repeatedly i.e. Immobilised enzymes. How to Immobilise Enzymes Physical methods: Adsorption: where enzymes are physically attached to inactive supports such as glass beads or cellulose particles. Trapped in a gel: sodium alginate is commonly used. This allows substrates in and products out but prevents the enzyme from leaving the gel. Enclosed by a membrane: when enzymes are kept within a membrane. How to immobilise enzymes Chemical Methods : Bonded to a support enzymes chemically bonded to a support such as glass beads or ceramics. Bonded to each other enzymes are chemically bonded to each other. Enzyme bonded to a support Advantages of Immobilised Enzymes • Efficiency of enzyme is not affected. • Immobilised enzymes can be easily recovered from the product so you can get a pure sample of product easily. • Immobilised enzymes can be reused this cuts costs. • Enzymes frequently become more stable when immobilised. Uses of Immobilised Enzymes Immobilised glucose isomerase converts glucose to fructose which is used to sweeten drinks. Lactase converts lactose to sweeter sugars glucose and galactose which are then used by food manufacturers. Penicillin acylase changes the structure of penicillin to make more antibiotics that will fight a wider range of bacteria. The Active Site The Active site is the part of the enzyme that combines with the substrate. The Active Site Contrary to belief the active site is not a rigid shape that is fixed to fit the substrate. When the substrate enters the active site it causes (or induces) it to change shape slightly. The enzyme then fits more precisely around the substrate this is known as the Induced Fit Model of enzyme action. The Bean Bag Theory The Induced Fit Model can be compared to the way a bean bag will adapt to fit snugly around our body shape when we sit in it. Mechanism of Enzyme Action The Induced Fit Model 1. The substrate combines with the active site of the enzyme 2. The active site is induced or caused to change shape slightly. Active Site Enzyme Substrate 3. Substrate and enzyme form an enzyme substrate complex. The bonds in the substrate are altered so that the substrate changes into the product(s). Enzyme Substrate complex Substrate changed to products which are released 4. The products leave the active site. The active site returns to its original shape and is ready for a new substrate molecule. Products Active Site Enzyme New Substrate Induced fit theory Denaturation • When most proteins are heated above 40⁰C or treated with certain chemicals or radiation they will gradually lose their 3 dimensional shape. • This means enzymes will not be able to form the enzyme substrate complex. • Denaturation: occurs when the shape of an enzyme is changed and it loses its biological activity. • Example: white of an egg is denatured when an egg is boiled. Energy Carriers • Energy is essential for cell metabolism e.g.: - Photosynthesis – energy in sunlight is used to make food. - Respiration – food is broken down to release energy. • ATP , NADP+ and NAD+ play a vital role in trapping and transferring energy in cellular activities. ADP and ATP ADP: • ADP is an abbreviation for Adenosine Diphosphate this is a molecule found in the cells of all organisms. • It is made of the base adenine , a 5 carbon sugar called ribose and 2 phosphate groups Unstable Bond Adenine Adenosine • ADP is a low energy molecule Ribose P P Diphosphate ADP and ATP ATP: • If another phosphate is added to ADP it forms ATP (Adenosine Triphosphate) Unstable Bond Adenine Ribose P P P • Extra energy is also added as there is an extra bond between the last two phosphate groups. • Addition of a phosphate group like this is called: Phosphorylation • ATP is rich in energy and stores this energy carrying it around in the cell i.e. Energy Carrier. ADP and ATP ATP: • ATP cannot store energy for very long it breaks down releasing energy and converting back to ADP. • Energy released is used to carry out most of the reactions in cells. • Most cells release energy from ATP 10 million times every second! NADP+ and NADPH NADP+: • NADP+ is a low energy molecule involved in photosynthesis. • NADP+ can combine with 2 high energy electrons and a proton (Hydrogen ion H+) to form NADPH. NADP+ + 2 electrons + H+ (LOW ENERGY) (HIGH ENERGY) NADPH (HIGH ENERGY) • Addition of electrons like this is called - Reduction • NADPH is a very high energy molecule. It’s energy is used to form glucose in photosynthesis. NADP+ and NADPH NADPH: • NADPH releases energy, in the form of two high – energy electrons, and a proton when broken down into NADP+. NADPH NADP+ (HIGH ENERGY) (LOW ENERGY) + 2 electrons + H+ (HIGH ENERGY) NAD+ and NADH • NAD+ is used in respiration • It can combine with 2 high energy electrons and a proton to form NADH which is very high in energy. • Remember P for photosynthesis, NADP+ is used in photosynthesis NAD+ in respiration.