Chemistry_History
advertisement

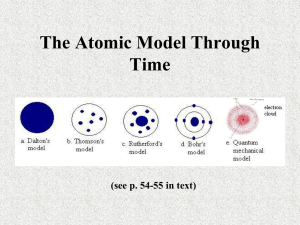
Brief History of Chemistry Chemistry from “Khemeia” • By 4000 BCE, in Egypt and Sumeria (Iraq), metals such as copper and gold were being used. • These were valuable because the metal could be shaped (malleable) and could keep an edge. • In addition to metallurgy Egypt also developed embalming and dying • The Greeks named this learning Khemeia from Khumos, juice of plants Democritus – all matter is made of small, indivisible particles called “atomos” Ancient Greeks • Democritus • 460 BCE to 370 BCE • was a student of Leucippus and co-originator of the belief that all matter is made up of various imperishable, indivisible elements • He called these atoma (sg. Atomon) or "indivisible units" Aristotle – matter is continuous and NOT made of smaller particles Ancient Greeks • Plato and Aristotle • Aristotle (384 BCE to 332BCE) • Believed there were 4 classic elements – Earth, fire, water, air • Alexander 323 BCE conquered Egypt. Under Ptolemy Greek-Egyptian Khemeia took hold. Mixed religion and learning. • Khemeia declined under the Romans who had no use for this mixture of mysticism and craft • 500 years of Arab dominated learning 650 - 1100 CE. Khemeia became AlChemi The “Age of Discovery” was start of true science. Age of Discovery • The discovery of the new world not described by the Greeks and improvements in navigation allowed Europeans to doubt the wisdom of the ancients and opened the way to the acceptance of new ideas. • The invention of the printing press in 1436 by Johann Gutenberg made the dissemination of new ideas to larger numbers possible. Alchemy not science Alchemy • ~ 1600 ACE • Mystical pseudoscience • Searched for “philosopher’s stone” • Some goals were transmutation, panacea and universal solvent 1st true “chemist” Discovered a relationship between pressure and volume (Boyle’s Law) Robert Boyle Developed the “scientific method” where experiments were devised to test theories. Defined an ‘element’ as something unable to be broken down into simpler substances. ~1660 1st Developed the Law of Conservation of Mass. Developed the theory of combustion. Determined the composition of water. Antoine Laurent Lavoisier Lavoisier was a French nobleman and chemist central to the 18th-century Chemical Revolution and a large influence on both the histories of chemistry and biology. He is widely considered to be the "Father of Modern Chemistry.” ~1770 Antoine Laurent Lavoisier It is generally accepted that Lavoisier's great accomplishments in chemistry largely stem from the fact that he changed the science from a qualitative to a quantitative one. Lavoisier is most noted for his discovery of the role oxygen plays in combustion. He recognized and named oxygen (1778) and hydrogen (1783). Lavoisier helped construct the metric system, wrote the first extensive list of elements, and helped to reform chemical nomenclature. He discovered that, although matter may change its form or shape, its mass always remains the same. Found that a given compound always contains exactly the same proportion of elements by mass - Law of Definite Proportions Joseph Louis Proust Joseph Louis Proust (September 26, 1754 – July 5, 1826) was a French chemist. He discovered the law of definite proportions, which states that every chemical compound contains fixed and constant proportions by weight of its constituent elements (law). Proust worked with many chemical compounds and still found that no matter where the compound came from or how it was produced, it had the same composition. The ratios of the masses of elements in a compound can always be reduced to small whole numbers – Law of Multiple Proportions John Dalton - 1800 If two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers. Atomic Theory John Dalton - Atomic Theory 1) all matter is composed of tiny particles called atoms 2) the atoms of an element are always identical while the atoms of different elements are different 3) compounds form when atoms combine; atoms combine in small whole number ratios 4) reactions involve reorganization of atoms; the atoms themselves do not change The ratios of the masses of elements in a compound can always be reduced to small whole numbers – Law of Multiple Proportions John Dalton –Billiard Ball Model John Dalton (1766 – 1844) proposed a basic model of the atom that helped establish many scientific concepts and also created the foundation for more modern models. His model suggested that atoms are the smallest particle of an element, that atoms of different elements have different masses, and that they are solid, indestructible units - much like a billiard ball. Equal volumes of gases at the same temperature and pressure contain the same number of molecules regardless of their chemical nature and physical properties. - Avogadro's Law Amadeo Avogadro - 1808 Avogadro's Law states that the relationship between the masses of the same volume of different gases (at the same temperature and pressure) corresponds to the relationship between their respective molecular weights. Hence, the relative molecular mass of a gas can be calculated from the mass of sample of known volume. The Avogadro constant (6.02214X×1023) is named after the early 19th century Italian scientist as an honor. Constructed first workable Periodic Table Dmitri Mendeleev - 1869 Constructed a periodic table by arranging elements: •in order of increasing atomic mass •in vertical groups based on similar chemical properties He left gaps for undiscovered elements and reversed the order of some elements to make their chemical properties fit. Lothar Meyer - 1870 Also constructed a periodic tablesimilar to that of Mendeleev Discovered electron (and +1 charge) Plum Pudding Model J. J. Thomson - 1897 Discovered that the negative components of atoms had the same mass regardless of which element they came from. Proposed the ‘plum pudding model of the atom where negative particles are dispersed throughout a positively charged atom. Marie Curie - 1903 Suggested that radioactive atoms were unstable and that energy was emitted during disintegration Discovered radium and polonium E = mc2 Albert Einstein Showed that mass and energy are interconvertible via: E = mc² Proposed the nucleus in an atom and that atoms are mostly empty space with electrons in that space Ernest Rutherford - 1911 Suggested that the atom was largely space with a very small but dense centre of positive charge called the nucleus Proposed that electrons orbited the nucleus like planets around the Sun Proposed Quantum Physics and electron energy shells Niels Bohr - 1913 Proposed that classical mechanics did not apply within the atom Proposed that electrons orbited the nucleus in shells of fixed energy Proposed different shaped electron orbitals Arnold Sommerfeld Expanded the Bohr model Electrons travel in orbitals, but the orbitals are not the same shape -- this leads to the electron cloud model of the atom Pauli’s Exclusion Principle says no two electrons do the exact same thing at the same time Wolfgang Pauli (1924) Predicted that electrons spin while orbiting the nucleus Proposed electron cloud Erwin Schrödinger - 1926 Developed the wave-like model of the electron and the charge cloud model of the atom No experiment can measure the position and momentum of a quantum particle simultaneously - Heisenberg’s Uncertainty Principle Werner Heisenberg The Heisenberg Uncertainty Principle states that for a very small particle, such as an electron, you cannot know both its exact momentum and its exact position at the same time. Discovered neutron James Chadwick - 1932 Discovered high energy particles with no charge and the same mass as the proton – the neutron