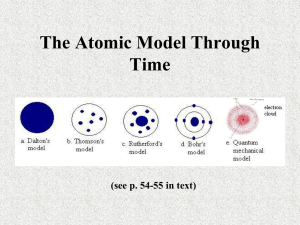
Atomic theory
advertisement

ATOMIC STRUCTURE Scientists that changed our view of the atom Democritus •Did not conduct any experiments •All matter is made of atoms •Atoms are indivisible and indestructible John Dalton John Dalton was born on September 6, 1766 in Cumbria, England. He provided us with the first concrete ideas about the atom. John Dalton – His Experiment John Dalton Four points to his theory: 1. 2. 3. 4. 5. All matter is composed of atoms that are indestructible and indivisible Atoms of one element are all the same; atoms of a different element are different (Au atoms are different from Ag atoms) Atoms cannot be changed into different atoms by chemical of physical changes (Pb atoms cannot be turned into Au atoms) All atoms are like Billiard Balls – a solid, sphere Atoms react in whole numbers JJ Thomson Joseph John Thomson was born in Cheetham Hill, a suburb of Manchester on December 18, 1856. He is given credit for the discovery of the electron. JJ Thomson – His Experiment Cathode Ray Tube JJ Thomson – His Experiment JJ Thomson 3 Points to his theory 1. 2. 3. * All gases had the same mass: charge ratio * All atoms contain small, charged particles called electrons * The atom now looked like “Plum Pudding” Goldstein Eugene Goldstein was born in 1850 in Germany. Goldstein – His Experiment Cathode Ray Tube Goldstein Major Point to his theory He is given credit for the discovery of a positively charged particle = Proton The atom was still thought to resemble Plum Pudding Millikan Robert Andrews Millikan was born on the 22nd of March, 1868, in Morrison, Ill. (U.S.A. ) Millikan – His Experiment Oil Drop Experiment Millikan 2 Points to his theory 1. All electrons have the same mass About 1/2000 atomic mass unit 2. All electrons have the same charge -1.6 x 10 -19 Coulomb The atom was still thought to resemble Plum Pudding Rutherford Ernest Rutherford was born August 30, 1871 in Nelson, New Zealand (one of 12 children) Rutherford – His Experiment Rutherford 3 Points to his theory 1. * Most alpha particle went through = Atom is mostly empty space (area of electrons) 2. * Some alpha particles bounced straight back = atom has a solid mass (nucleus) 3. * Some alpha particles reflected = nucleus is positively charged New Model = Nuclear Model Schrödinger Erwin Schrödinger born August 12 in Vienna, Austria in 1887. Schrödinger – His Experiment Schrödinger Major points 1. Electrons do not follow fixed paths 2. They move randomly in areas of probability (orbitals) 3. There are specific energies associated with each orbital New Model = Quantum Mechanical Model Chadwick James Chadwick was born in Cheshire, England, on 20th October, 1891. Chadwick – His Experiment Chadwick 2 Points to his theory 1. 2. Discovered the Neutron – Same mass as a proton, but without a charge Mass could be converted into energy Model was still thought to resemble the Quantum Mechanical Model