Ch 2 2 Minerals
advertisement
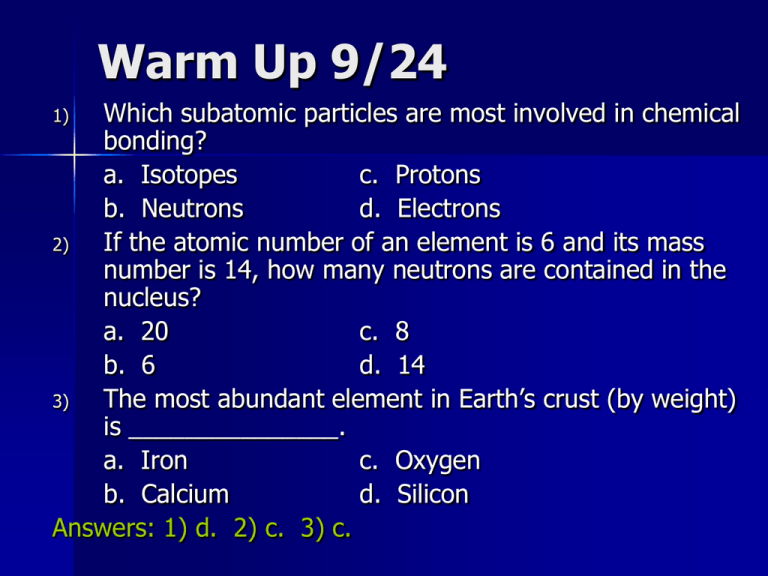
Warm Up 9/24 Which subatomic particles are most involved in chemical bonding? a. Isotopes c. Protons b. Neutrons d. Electrons 2) If the atomic number of an element is 6 and its mass number is 14, how many neutrons are contained in the nucleus? a. 20 c. 8 b. 6 d. 14 3) The most abundant element in Earth’s crust (by weight) is _______________. a. Iron c. Oxygen b. Calcium d. Silicon Answers: 1) d. 2) c. 3) c. 1) Minerals Chapter 2, Section 2 Minerals Mineral – naturally occurring, inorganic solid with an orderly crystalline structure and a definite chemical composition Minerals must have the following characteristics: 1. Naturally Occurring 2. Solid Substance 3. Orderly Crystalline Structure 4. Definite Chemical Composition 5. Generally Considered Inorganic Minerals How Minerals Form There are four major processes by which minerals form: crystallization from magma, precipitation, changes in pressure and temperature, and formation from hydrothermal solutions. Crystallization from Magma As magma cools, elements combine to form minerals. Precipitation As water evaporates, some of the dissolved substances can react to form minerals. Changes in water temperature may also cause dissolved substances to precipitate out of a body of water. Pressure and Temperature Crystals form when existing minerals are subjected to changes in pressure and temperature. Hydrothermal Solutions A very hot mixture of water and dissolved substances. Come into contact with existing minerals and the chemical reactions between them form new minerals Concept Check Describe what happens when a mineral is subjected to changes in pressure or temperature. The mineral often becomes unstable, and its atoms react to form a new mineral Mineral Groups Common minerals, together with the thousands of others that form on Earth, can be classified into groups based on their composition Silicates – Silicon and oxygen combine to form a structure called the silicon-oxygen tetrahedron Carbonates – Minerals that contain the elements carbon, oxygen, and one or more other metallic elements Oxides – Minerals that contain oxygen and one or more other elements, which are usually metals Sulfates and Sulfides – Minerals that contain the element sulfur Halides – Minerals that contain a halogen ion plus one or more other elements Native Elements – Group of minerals that exist in relatively pure form Silicates Common Silicate Minerals Carbonates Oxides Sulfates and Sulfides Halides Native Elements Concept Check What is the silicon-oxygen tetrahedron, and in how many ways can it combine? The silicon-oxygen tetrahedron consists of one silicon atom and four oxygen atoms and provides the framework of every silicate mineral. These tetrahedra can join to form single chains, double chains, sheets, and three-dimensional networks. In these arrangements the corner oxygen atoms are shared between silicon atoms so the ratio is not necessarily 1 to 4. Assignment Read Chapter 2, Section 2 (pg. 44-49) Do Section 2.2 Assessment #1-7 (pg. 49)