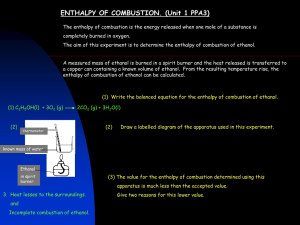
Production of materials
advertisement

Production of materials Ethylene (ethene) Although ethylene is a widely used raw material very little of it is found in either natural gas or crude oil. Instead it has to be produced from other hydrocarbons by a process called cracking. Cracking: process in which large hydrocarbons are broken down into smaller ones with the help of heat and/or a catalyst. Cracking During the cracking process bonds within the hydrocarbon molecule are broken. Ethene is produced in one of two ways: From crude oil by catalytic cracking of fractions from the distillation column From natural gas by thermal cracking One possible reaction involving the cracking of hydrocarbon C15H32 might be http://www.chemguide.co.uk/organicprops/alkanes/cracking.html Reactions of ethane and ethene What do ethane and ethene have in common? 2 carbon atom chain, non-polar (insoluble in water, low melting and boiling points, both undergo combustion with oxygen How do they differ? ethene has a double bond and therefore is much more reactive Reactions of ethene Like all alkenes, ethene undergoes addition reactions. Why? Answer: When the double bond is broken additional atoms or groups of atoms can be added – one to each C atom previously linked by the double bond. Addition of hydrogen Addition of hydrogen to ethene results in the formation of ethane. Ethylene + CH2= CH2 (g) + hydrogen H2 (g) Alkene + hydrogen ethane CH3-CH3 (g) alkane Addition of halogens Addition of a halogen (eg: Cl, Br) to ethene results in the formation of a haloalkane ethene + bromine CH2= CH2 (g) + Br2 (l) Alkene + halogen 1,2 dibromoethane CH2Br-CH2Br (g) di-halo-alkane Addition of hydrogen halides Addition of a hydrogen halides (eg: HCl) to ethylene also produces haloalkanes. Ethylene + hydrogen chloride chloroethane CH2= CH2 (g) + CH3-CH2Cl(l) HCl (g) Alkene + hydrogen halide haloalkane Addition of water Addition of water (in the presence of an acidic catalyst) to ethylene produces an ethanol Ethylene + water ethanol CH2= CH2 (g) + H2O (l) CH3-CH2OH (l) Alkene + water alkanol Reactions of alkanes 1. Combustion reaction: Alkanes burn in air to produce CO2 and H2O C3H8 (g) + 5O2 (g) propane + oxygen Alkane + oxygen 3CO2 (g) + 4H2O (g) carbon + water dioxide carbon + water dioxide Reactions of alkanes 1. Substitution reaction: Alkanes react with Cl2, Br2, I2 (halogens) when exposed to ultraviolet light C6H14 (l) + Br2 (l) hexane + bromine Alkane + halogen C6H13Br(l) + HBr(aq) bromohexane haloalkane Polymerisation Reaction in which many small molecules (monomers) combine to form one large molecule (polymer). There are two main types of polymerisation reactions: Addition polymerisation Condensation polymerisation Addition polymerisation In the process of addition polymerisation monomers simply add together without the loss of any atoms. Basically the double bond opens out to form single bonds with neighbouring molecules. Addition reactions involve unsaturated hydrocarbons. Addition polymerisation Condensation polymerisation Condensation polymerisation involves a reaction between two monomers which have different functional groups. Small molecules such as water are eliminated during this reaction. Carboxylic acid functional group COOH Condensation polymerisation Amine functional group –NH 2 Alcohol functional group -OH Synthetic polmers Ethene is the simplest monomer capable of undergoing addition polymerisation. Some important synthetic polymers formed from ethene include: Poly(ethene) (polyethelyne) Poly (vinyl chloride) PVC Poly (styrene) Poly (acylonitrile) PAN Poly (propene) (polypropylene) Biopolymers Polymers produced by living organisms are called biopolymers. Examples include: Cellulose Starch Proteins Nucleic acids Alcohols Alcohols are a family of carbon compounds that contain the hydroxy group (-OH). Alkanols are a specific group of alcohols where one or more hydrogen atoms in an alkane are replaced by an –OH functional group. Alkanols are represented by the general formula ROH where R = alkyl group Ethanol Alcohols : Nomenclature Add the suffix ‘ol’ in place of the ‘e’ on the name of the hydrocarbon to which the –OH group is attached. A number indicates the position of the carbon atom containing the -OH group. If there are more than one –OH group add the suffixes ‘-diol’, ‘-triol’ and so on. Ethanol 1,2 ethanediol Primary alcohol The carbon atom attached to the _OH group has two carbon atoms bonded to it. Secondary alcohol The carbon atom attached to the _OH group has two carbon atoms bonded to it. Tertiary alcohol The carbon atom attached to the _OH group has two carbon atoms bonded to it. Ethanol as a solvent Ethanol is a good solvent because it is a very polar molecule. When ethanol and water are mixed they readily dissolve in each other. This is due to the polar nature of the O-H bond. C δ+ O δHδ+ The polar end of the ethanol molecule interacts with other polar molecules to form dipole-dipole forces or hydrogen bonds eg: with water Ethanol as a solvent Ethanol and hexane (a non-polar molecule) readily dissolve in each other. The non - polar end of the ethanol molecule (the alkyl chain) forms dispersion forces with other non-polar molecules. This enables ethanol to act as a solvent for some non-polar molecules. Production of Ethanol 1. Hydration of ethanol: industrial ethanol is produced by the acid catalysed addition of water to ethene, represented by the equation: CH2 = CH2 (g) + H20 (g) CH3 - CH2OH(g) Production of ethanol 2. Fermentation: process in which glucose is broken down to ethanol and carbon dioxide by the action of enzymes in yeast (these act as catalyst). C6H12O6 (aq) 2CH3 - CH2OH (aq) +2CO2 (g) This process is exothermic. Ethanol as a fuels The combustion of ethanol is an exothermic reaction. C2H5OH(g) + 3O2 2CO2(g) + 3H2O(g) The amount of heat released can be expressed as the molar heat of combustion: ‘Heat liberated on complete combustion of one mole of a substance’ Calorimetry Calorimetry is a method used to determine heat of combustion. Essentially we measure the change in temperature of measured mass of water heated by the combustion of a measured amount of fuel. This is then used to calculate heat energy release per mole of substance burned. Molar heat of combustion 1. Find the mass of the fuel burned ??? by weighing the fuel and container before and after heating 2. Calculate the moles of fuel burned ??? n= m/M Molar heat of combustion 3. From the rise in water temperature calculate heat produced by combustion of that many moles of fuel??? ΔH = m C ΔT 4. Calculate how much heat could have been produced by one mole of the substance Oxidation-reduction Reactions which involve the transfer of electrons are called oxidation-reduction reactions. OXIDATION = LOSS OF ELECTRONS REDUCTION = GAIN OF ELECTRONS Zn (s) + 2HCl (aq) Oxidation: Zn (s) Reduction: 2H+(aq) + 2e- ZnCl2 (aq) + H2 (g) Zn 2+ (aq) + 2eH2 (g) Displacement reactions Displacement reactions are oxidation-reduction reactions in which a metal converts the ion of another metal to the neutral atom. In these reactions the metal dissolves and the ions of the other metal are reduced to elemental metal and deposit out of solution. Example: Cu 2+ ions have a greater tendency to gain electrons than Zn 2+ ions. As a result there is a transfer of electrons from the Zn metal to the Cu (II) ions. As the reaction proceeds Zn metal dissolves and goes into solution as Zn ions and Cu metal is formed The activity series The activity series can be used to predict whether a metal will displace the ions of another metal. K>Na>Mg>Al>Zn>Fe>Sn>Pb>Cu>Ag>Hg>Pt>Au The more reactive metal will displace another metal from a solution of its ions. Oxidation states In many oxidation-reduction reactions it is not obvious which species has been reduced and which has been oxidised. To overcome this problem we use a system of assigning oxidation states to atoms to keep track of the number of electrons transferred or shared in oxidation-reduction reactions. Oxidation state is an arbitrary number assigned according to a set of rules. Rules for determining oxidation state 1. 2. 3. 4. 5. Uncombined elements have an oxidation state of 0 Ions have an oxidation state equal to their charge (eg: Na+ = +1) Oxygen in compounds has a charge of -2 in oxides and -1 in peroxides Hydrogen in compounds has a charge of +1 when combined with non-metals and -1 when combined with metals The oxidation state of a compound or polyatomic ion is the sum of the oxidation states of all its atoms. Oxidation state Note that the number of electrons lost or gained = change in oxidation state Oxidation involves an increase in oxidation state. Half equation Zn (s) Zn 2+ (aq) + 2eOxidation state 0 2+ Reduction involves a decrease in oxidation state. Half equation: 2H+(aq) + 2eOxidation state: 1+ H2 (g) 0 Electrochemical cells Redox reactions can be used to generate electricity in a galvanic cell Example: When zinc metal is placed in CuSO4 solution, following reaction take place: Zn(s) + CuSO4(aq) ZnSO4(aq) + Cu(s) Oxidation: Zn(s) → Zn+2 + 2eReduction: Cu+2 + 2e- → Cu Overall: Zn(s) + Cu+2 → Zn+2 + Cu(s) Galvanic cell Each of the two parts of the cell is called a half cell.Each half cell is connected by a salt bridge which completes the circuit and allows ions to travel between each half cell. How does the galvanic cell work? Zn loses electrons to form Zn ions in solution. The Zn strip dissolves Zn ions travel through the external circuit to the Cu strip where they are accepted by the Cu ions The Cu ions are reduced to Cu atoms which deposit on the strip How does a galvanic cell work? As reaction continues excess Zn2+ ions build up in the ZnSO42solution excess negative SO42- ions build up in the CuSO42- solution To maintain electrical neutrality in the half cell solutions positive Na+ ions move into the copper half cell from the salt bridge at the same time NO3- ions move into the zinc half cell Standard Reduction potentials Standard reduction potential (EO) is a measure of the relative tendency of a substance to gain one or more electrons compared to the standard hydrogen half cell. The larger the EO value the greater the oxidising power of a substance e.m.f or voltage of a galvanic cell is the difference in the reduction potentials of the two couples making up the cell.