types of mixtures notes
advertisement

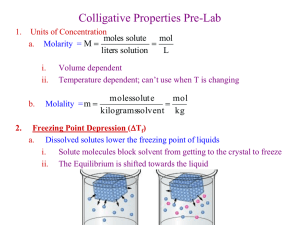
Types of Mixtures Heterogeneous vs. Homogeneous Solutions A homogeneous mixture of 2 or more substances in a single phase. Solvent: the dissolving medium Solute: the substance which is dissolved Types of Solutions: (particle size is small, 0.01-1nm) oxygen in nitrogen, carbon dioxide in water water in air, alcohol in water, mercury in silver/tin sugar in water, zinc in copper (brass) Other Types of Mixtures Suspensions: Particles in a solvent are so large, >1000 nm, they will settle out unless stirred. Colloids: Particles are between 1-1000 nm. They are small enough to be suspended throughout the solvent. TABLE 13.2 Tyndall Effect: scattering of light by colloidal particles. Solutes Electrolytes: Dissolve in water to give a conducting solution. Ex: NaCl Nonelectrolytes: Dissolves in water to give solution that does NOT conduct eleectricity. Ex: C12H22O11 The Solution Process 1. Factors Affecting the Rate of Dissolving: Surface Area Agitation Heat 2. Solubility: the amount of a substance required to form a saturated solution at a given temp. Types of Solution Saturated: contains max amount of dissolved solute Unsaturated: contains less solute than saturated solnution. Supersaturated: contains more solute than a saturated solution under the same conditions. Like dissolves Like Predicts whether one substance will dissolve another: Polarity of molecule Intermolecular forces between solute/solvent. Hydration: Solution process with water as solvent. Ionic compounds are not normally soluble in nonpolar solvents (CCl4). Why?? Liquid Solutes/Solvents Immiscible: Liquid solute/solvent are NOT soluble in each other. Ex: Oil/Water Miscible: Liquid solute/solvent are soluble in each other. Ex: Oil/Gasoline Nonpolar molecules exert no strong attractive/repulsive forces- molecules mix freely. Effects of Pressure on Solubility Very little effect on liquids or solids Solubility of a gas is unchanged at a given pressure: gas + solvent solution Henry’s Law: The solubility of a gas in a liquid increases as the pressure of that gas on the surface of the liquid increases. Effervescence: The rapid escape of a gas from a liquid. Effects of Temperature on Solubility Gases Inverse relationship: An increase in temperature, decreases gas solubility. Why? More solute molecules can escape the attraction of solvent molecules. Liquids and Solids Difficult to predict Heats of Solution The net amount of heat energy absorbed or released when a specific amount of solute dissolves in a solvent. Overhead Transparency #74 - Hsolution = Heat is released = Steps 1 and 2 < Step 3 Hsolution = Heat is absorbed = Steps 1 and 2 > Step 3. + Concentrations of Solutions A measure of the amount of solute in a given amount of solvent or solution. Molarity = moles solute/ Liter of solution Molality = moles solute/ kg solvent Doesn’t change with temperature changes. QUESTION: Answer in your notebook. You are asked to prepare 5.00 Liters of a 2.00M solution of Sodium Acetate. How many grams of sodium acetate would you measure out? Given all of the necessary glassware, what steps would you take to make your solution? Compounds in Aqueous Solution Dissociation: Separation of ions when dissolved. NaCl (s) Na+ (aq) + Cl – (aq) Precipitation Reactions: Compounds of very low solubility…Practically Insoluble. Table 14. 1 shows us general guidelines to predict solubility. Dissociation reactions are NOT written for insoluble compounds. Net Ionic Equations Includes only those compounds/ions that undergo a chemical change in a reaction in an aqueous solution. 1. Convert chemical equation ionic equation 2. Cancel out spectator ions (ions that do not take part in a chemical reaction). Ex: Sodium Chloride + Silver Nitrate Ionization The term used for the formation of ions from molecular compounds. (The creation of ions from where there are none) Degree of ionization depends on strength of bonds between solute AND strength of attraction between solute/solvent. Ex: HCl (g) + H2O (l) H3O+ (aq) + Cl - (aq) Hydronium Ion Strong vs. Weak Electrolytes Strong Electrolyte: A compound whose dilute aqueous solution conducts electricity well because of the high presence of ions when dissolved. Ex: HCl, NaCl Weak Electrolytes: Solution does NOT conduct electricity well because there is a low presence of ions that are dissolved. Ex: HF, CH3COOH Colligative Properties Properties that depend on the amount of solute particles and not their identity Vapor Pressure Lowering: The vapor pressure of water containing sugar (or any other nonvolatile solute) is less than the vapor pressure of pure water. Concentration of water WHY? molecules is lower at the surface of the liquid b/c of increased attraction between solute/solvent. Boiling Point Elevation The boiling point of water containing a nonvolatile solute is higher than the boiling point of pure water. WHY? Since the vapor pressure is lower, water particles need a higher KE to overcome atmospheric pressure and boil. Freezing Point Depression The freezing point of a solution is lower than the freezing point of a pure solvent. WHY? Solute particles get in the way of water particles trying to freeze, so water particles need to move slower to ensure the correct orientation of the lattice. Freezing & Boiling Point Constants Δ tf = Kf m Freezing point depression: The difference between the freezing point of a pure solvent and solution Freezing Point Constant: The freezing point depression of the solvent in a 1-molal solution (°C/m) Molality Δ tb = Kb m Boiling Point Elevation Boiling Point Constant (Table 14.2) Molality Osmotic Pressure The external pressure required to stop osmosis. Osmosis: The movement of solvent through a semipermeable membrane from low high solute concentration. Osmotic Pressure increases with the number of solute particles in solution. Electrolytes and Colligative Properties Colligative Properties depend on the AMOUNT of particles produced in solution. 1m solution of NaCl produced more than 1m solution of dextrose due to NaCl dissociation. Table 14.3 QUESTION: Write your answer on a sheet of paper and hold up when asked Which would produce the greatest change in freezing/boiling points? a. b. c. d. 1m solution Sucrose (C12H22O11) 1m solution Glucose (C6H1206) 1m solution KCl 1m solution of CaCl2 ANSWER: D. More ions are produced in solution.