File - Thomas Tallis Science
advertisement

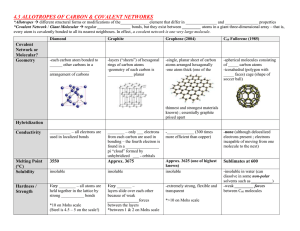
Structures and properties You must learn the following: The Structure and properties of: • Giant Ionic lattices eg, Sodium chloride • Simple molecular covalent compounds • Giant covalent structures eg, diamond and graphite, Silicon dioxide Giant ionic lattices, eg sodium chloride Ions form a regular pattern due to positive and negative charges attracting Ionic bonds are strong causing high melting and boiling point Ionic compounds conduct electricity when molten (or dissolved in water) because ions are free to move and carry the current Simple molecular covalent compounds Properties of simple molecular covalent substances: • Strong covalent bonds between atoms • Weak forces between molecules causing low melting and boiling points • Do not conduct electricity because molecules have no overall electrical charge Giant Covalent Structures eg diamond Each carbon atom in diamond has 4 strong covalent bonds so is very hard and has a high melting point and boiling point Giant covalent structures eg, Graphite • Each carbon atom bonds to 3 other atoms forming layers which are free to slide over each other • Higher tier: one electron from each carbon is delocalised and is free to move and carry current Silicon dioxide Sand is mostly made of the mineral quartz, which is silicon dioxide. It has a giant covalent structure made up of silicon and oxygen atoms Each silicon atom (2.8.4) is bonded to four oxygen atoms, and each oxygen atom (2.6) is bonded to two silicon atoms. O Si O O O Bonding 7 of 45 © Boardworks Ltd 2007 Bonding summary The properties of polymers depend on what they are made from and the conditions they are made For example, low density (LD) and high density (HD) poly(ethene) are produced using different catalysts and reaction conditions Thermo softening polymers consist of individual, tangled polymer chains with cross-links between them so that they do not melt when they are heated Nanoscience refers to structures that are 1-100nm in size, in the order of a few hundred atoms Nanoparticles show different properties to the same materials in bulk and have a high surface area to volume ratio This may lead to the development of new computers, new catalysts, new coatings, highly selective sensors, stronger and lighter construction materials and new cosmetics such as sun tan creams and deodorants Paper chromatography • Elements and compounds can be detected and identified using instrumental methods. • Instrumental methods are accurate, sensitive and rapid and are particularly useful when the amount of a sample is very small. • Chemical analysis can be used to identify additives in foods. • Artificial colours can be detected and identified by paper chromatography Explain: What does the picture show? Gas chromatography mass spectroscopy Gas chromatography linked to mass spectroscopy (GC-MS) is an example of an instrumental method • Different substances, carried by a gas, travel through a column packed with a solid material at different speeds, so that they become separated • The number of peaks on the output of a gas chromatograph shows the number of compounds present • The molecular mass is given by the molecular ion peak • The position of the peaks on the output indicates the retention time • A mass spectrometer can identify substances very quickly and accurately and can detect very small quantities • Gas chromatography