Cell Membranes Osmosis and Diffusion
advertisement

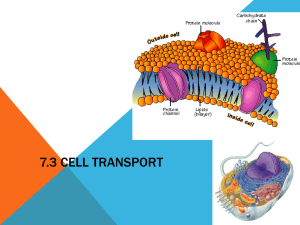
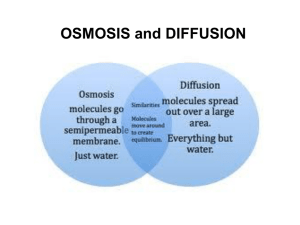
Cell Membranes and Methods of Transport How Do Molecules Move? • Stand with your classmates in locations that are evenly spaced throughout the classroom. • Your teacher will spray an air freshener into the room. When your first smell the air freshener, raise your hand. • Note how long it takes for other students to smell the scent. How Do Molecules Move? Developing a Hypothesis How was each student’s distance from the teacher related to when he or she smelled the air freshener? Develop a hypothesis about why this pattern occurred. Key Concepts • How do most small molecules cross the cell membrane? • Why is osmosis important in cells? • What is the difference between passive transport and active transport? Functions of Membranes 1. 2. 3. 4. Protect cell Control incoming and outgoing substances Maintain ion concentrations of various substances Selectively permeable - allows some molecules in, others are kept out Phospholipid Bi-layer Selectively Permeable Membrane • Oxygen, food molecules and waste products all must pass through the cell membrane. • Substances that can move into or out of a cell do so by one of four methods: Diffusion Osmosis Facilitated Transport Active Transport Fluid Mosaic Model Methods of Transport Across Membranes 1. Diffusion -passive transport of solutes - no energy expended 2. Osmosis - Passive transport of water across membrane – no energy expended 3. Facilitated Diffusion - Use of proteins to carry polar molecules or ions across 4. Active Transport- requires energy to transport molecules against a concentration gradient – energy is in the form of ATP Types of Transport Diffusion • Diffusion is the main method by which small molecules move across the cell membrane. • It is the process by which molecules tend to move from an area of high concentration to an area of low concentration. • Diffusion is caused by molecules moving and colliding. • Molecules move from one side of a membrane to another, unfacilitated, depending on the concentration. Diffusion of liquids Diffusion • Diffusion is the net movement of molecules (or ions) from a region of their high concentration to a region of their lower concentration. The molecules move down a concentration gradient. Molecules have kinetic energy, which makes them move about randomly. As a result of diffusion molecules reach an equilibrium where they are evenly spread out. This is when there is no net movement of molecules from either side. Diffusion of Bromine Diffusion through a membrane Cell membrane Inside cell Outside cell Diffusion through a membrane Cell membrane diffusion Inside cell Outside cell Diffusion through a membrane Cell membrane Inside cell Outside cell EQUILIBRIUM What determines the rate of diffusion? There 4 factors: 1. The steepness of the concentration gradient. The bigger the difference between the two sides of the membrane the quicker the rate of diffusion. 2. Temperature. Higher temperatures give molecules or ions more kinetic energy. Molecules move around faster, so diffusion is faster. 3. The surface area. The greater the surface area the faster the diffusion can take place. This is because the more molecules or ions can cross the membrane at any one moment. 4. The type of molecule or ion diffusing. Large molecules need more energy to get them to move so they tend to diffuse more slowly. Non-polar molecules diffuse more easily than polar molecules because they are soluble in the non polar phospholipid tails. Molecules that diffuse through cell membranes Oxygen – Non-polar so diffuses very quickly. Carbon dioxide – Polar but very small so diffuses quickly. Water – Polar but also very small so diffuses quickly. Osmosis • The diffusion of water molecules through a selectively permeable membrane is called osmosis. • Because cells cannot function properly without adequate water, many cellular processes depend on osmosis. DILUTE SOLUTION Osmosis CONCENTRATED SOLUTION Cell membrane partially permeable. Sugar molecule VERY Low conc. of water molecules. High water potential. VERY High conc. of water molecules. High water potential. Inside cell Outside cell Osmosis Cell membrane partially permeable. OSMOSIS High conc. of water molecules. High water potential. Inside cell Low conc. of water molecules. High water potential. Outside cell Osmosis Cell membrane partially permeable. OSMOSIS Inside cell Outside cell EQUILIBRIUM. Equal water concentration on each side. Equal water potential has been reached. There is no net movement of water Salt Sucks • Water will move in the direction where there is a high concentration of solute (and hence a lower concentration of water. • A simple rule to remember is: Salt Sucks • Salt is a solute, when it is concentrated inside or outside the cell, it will draw the water in its direction. This is also why you get thirsty after eating something salty. Passive Transport • The movement of dissolved materials through a cell membrane without using cellular energy is called passive transport. • Diffusion and osmosis are both types of passive transport. DIFUSSION LAB • Open Lab that was emailed to you. • Lets discuss this. Link Facilitated Difussion Facilitated diffusion • Large polar molecules such as glucose and amino acids, cannot diffuse across the phospholipid bilayer. Also ions such as Na+ or Clcannot pass. • These molecules pass through protein channels instead. Diffusion through these channels is called FACILITATED DIFFUSION. • Movement of molecules is still PASSIVE just like ordinary diffusion, the only difference is, the molecules go through a protein channel instead of passing between the phospholipids. Facilitated Diffusion through a membrane Cell membrane Protein channel Inside cell Outside cell Facilitated Diffusion through a membrane Cell membrane diffusion Protein channel Inside cell Outside cell Facilitated Diffusion through a membrane Cell membrane diffusion Protein channel Inside cell Outside cell EQUILIBRIUM Facilitated Diffusion: Molecules will randomly move through the opening like pore, by diffusion. This requires no energy, it is a PASSIVE process. Molecules move from an area of high concentration to an area of low conc. Lactose intolerance Active Transport Active Transport • When a cell needs to take in materials that are in higher concentration inside the cell than outside the cell, the movement of the materials requires energy. • Active transport is the movement of materials through a cell membrane using cellular energy (ATP). • The main difference between passive and active transport is that active transport requires the cell to use its own energy and passive transport does not. Active Transport • Cells have several ways of moving materials by active transport. • In one method, transport proteins in the cell membrane ‘pick up’ molecules outside the cell and carry them in. • Another method of active transport is engulfing, in which the cell membrane wraps around, or engulfs, a particle and forms a vacuole within the cell. Vesicles and the Cell Membrane • • • • Endocytosis is the case when a molecule causes the cell membrane to bulge inward, forming a vesicle. Phagocytosis is the type of endocytosis where an entire cell is engulfed. Pinocytosis is when the external fluid is engulfed. Receptor-mediated endocytosis occurs when the material to be transported binds to certain specific molecules in the membrane. Examples include the transport of insulin and cholesterol into animal cells. The opposite of endocytosis is exocytosis. Large molecules that are manufactured in the cell are released through the cell membrane. Receptor Proteins These proteins are used in intercellular communication. In this animation you can see the a hormone binding to the receptor. This causes the receptor protein release a signal to perform some action. Carrier Proteins These are carrier proteins. They do not extend through the membrane. They bond and drag molecules through the bi-lipid layer and release them on the opposite side. Vesicle-mediated transport • Vesicles and vacuoles that fuse with the cell membrane may be utilized to release or transport chemicals out of the cell or to allow them to enter a cell. • Exocytosis is the term applied when transport is out of the cell. Overview of Transport Systems Most Cells are Small • One reason is related to the fact that all materials move into and out of the cell membrane. • Once a molecule enters a cell, it is carried to its destination by a stream of moving cytoplasm. • In very large, streams of cytoplasm must travel farther to carry materials from the cell membrane to all parts of the cell. Solutions • Solutions are made of solute and a solvent • Solvent - the liquid into which the solute is poured and dissolved. We will use water as our solvent today. • Solute - substance that is dissolved or put into the solvent. Salt and sucrose are solutes. Tonicity is a relative term • Hypotonic Solution • Hypertonic Solution • Isotonic Solution Isotonic • If the concentration of solute (salt) is equal on both sides, the water will move back in forth but it won't have any result on the overall amount of water on either side. • "ISO" means the same Hypotonic Solution • The word "HYPO" means less, in this case there are less solute (salt) molecules outside the cell, since salt sucks, water will move into the cell. • The cell will gain water and grow larger. In plant cells, the central vacuoles will fill and the plant becomes stiff and rigid, the cell wall keeps the plant from bursting • In animal cells, the cell may be in danger of bursting, organelles called CONTRACTILE VACUOLES will pump water out of the cell to prevent this. Hypertonic Solutions • The word "HYPER" means more, in this case there are more solute (salt) molecules outside the cell, which causes the water to be sucked in that direction. • In plant cells, the central vacuole loses water and the cells shrink, causing wilting. • In animal cells, the cells also shrink. • In both cases, the cell may die. • This is why it is dangerous to drink sea water - its a myth that drinking sea water will cause you to go insane, but people marooned at sea will speed up dehydration (and death) by drinking sea water. • This is also why "salting fields" was a common tactic during war, it would kill the crops in the field, thus causing food shortages. Plant and Animal Cells put into various solutions Effects of Plant Cells in Different Solutions RBC’s put in Various Solutions Tonicity is a relative term • Hypotonic Solution – One solution has a lower concentration of solute than another. • Hypertonic Solution – One solution has a higher concentration of solute than another. • Isotonic Solution Both solutions have same concentrations of solute. Math Skills: Ratios The concentration of a solution can be expressed as a ratio. A ratio compares two numbers. It tells you how much you have of one item in comparison to another. For example, suppose you dissolve 5 g of sugar in 1 L of water. You can express the concentration of the solution in ratio form as 5g : 1L, or 5g/L. Practice Suppose you dissolve 7g of salt in 1L of water. Express the concentration of the solution as a ratio.