Enzymes (PowerPoint) - Akkad`s BC Biology 12
advertisement

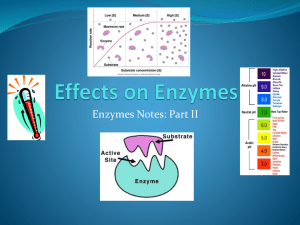
BC BIOLOGY 12 Enzymes Metabolism: is the total of all the chemical reactions in an organism. • All of life’s metabolic reactions require enzymes. • Each of these metabolic reactions typically occur in a series of chemical reactions called Metabolic Pathways. Metabolic pathways can be classified into two groups: 1. Catabolic Pathways: involves the breakdown or degradation of large biological molecules usually with the release of energy. • Cellular respiration degrades glucose to CO2 and H2O and releases energy. 2. Anabolic Pathways: • involves the construction of biological molecules using energy. • Photosynthesis: uses CO2 and H2O with light energy to make glucose. Chemical Reactions between Plant and Animal cells Enzymes • Enzymes allow metabolic reactions to occur without investing a lot of energy. • Enzymes lower the activation energy for chemical reactions to take place. • Enzymes help build & break bonds by chemically bonding to the reactants to form a transition “enzyme - substrate complex” which is highly unstable and therefore reactive. • Enzymes act as catalysts which allow lowering the Eact of metabolic reactions. Enzymes Structure • They do this because enzymes have a highly specific 3D shape that includes a place on the enzyme which is exactly complementary in shape to the shape of the substrate. • This is called the “Active Site” and it is much like a lock and key. Enzymes Structure • Enzymes are highly specific. • One type of enzyme can catalyze only one type of reaction. • This is due to the highly specific shape of the active site that will “fit” only one specifically shaped substrate. Because the substrate attachment is temporary only, enzymes can be reused thousands of time therefore they work in very low concentrations in cells. • This saves cells lots of energy they would have had to spend building many enzyme copies. Enzymes Structure • Enzymes are highly specific. • One type of enzyme can catalyze only one type of reaction due to the highly specific shape of the active site that will “fit” only one specifically shaped substrate. • Since the substrate attachment is temporary only, enzymes can be re-used over and over again,saving cells a lot of energy of remanufacturing of enzymes. • Many enzymes are named after the substrate. sucrose is broken down by sucrase lipids / lipase amylose (starch ) amylase fructose / fructase peptides / peptidase urea / urease lactose / lactase RNA / ribonuclease DEGRADATIVE REACTION Degradation or hydrolysis is when a substrate is broken down into products, with the help of an enzyme and water. Catabolic Reaction DEGRADATIVE REACTION Below is an example of a HYDROLYTIC REACTION where water is added to ENZYME SUBSTRATE COMPLEX to break the bond between sucrose (dissacharide) to produce two (monosccharides) fructose and glucose. Catabolic Reaction SYNTHETIC REACTION Synthesis is when two reactants are bonded together to form a product. Anabolic Reaction Enzymes Structure • Some enzymes are not constructed of pure protein but include another piece. 1. Cofactors: these are non organic atoms or molecules which complete the shape and therefore reactivity of an enzyme. • ex. zinc, copper, and other ions Enzymes Structure 2. Coenzymes: these are organic additions to the protein portion (called the apoenzyme) that completes the enzyme. • Coenzymes are vital to life as they bring numerous enzymes to full functional ability. Vitamins act as co-enzymes. Enzyme Reaction Rates • Numerous factors control the rate at which enzyme catalyzed reactions occur. 1. Substrate concentration: reaction rate increase as the concentration of the reactant increase up to a point where the rate levels off. • This is because every active site of every enzyme is occupied with a reactant molecule. • The enzymes are working at full capacity. Effect of Substrate Concentration Enzyme Reaction Rates 2. Enzyme concentration: Reaction rate will increase as enzyme concentration increases until the reactants run out, then the rate slows and finally stops. 3. Temperature: enzymes have an optimum operating temperature near body temperature. • Too hot or too cold can either temporarily or permanently denature enzymes (proteins). Effect of Temperature Enzyme Reaction Rates 4. pH: Enzymes have an optimum pH range which is usually quite narrow and centers around pH 7 (are exceptions). • Reaction rate slows as the pH changes in either direction. Effect of pH Enzyme Reaction Rates • 5. Competitive Inhibitors: these are molecules that enter a cell which are 3D shaped very near to an enzymes substrate. These mimic the substrate and attach to the enzymes active site but do nothing but occupy it. • The site is now unavailable to the real substrate so the reaction slows. Competitive Inhibitor Enzyme Reaction Rates 6. Noncompetitive Inhibitors: • These are molecules that attach to an enzyme at some place other than the active site (Allosteric site) • By so doing, they distort the 3D shape of the active site such that it can’t attach its substrate. • Ex. Heavy metals, antibiotics, poisons, pesticides and herbicides. Noncompetitive Inhibitor Allosteric site NEGATIVE FEEDBACK or FEEDBACK INHIBITION ATP • When a terminal phosphate breaks off, free energy is released and available for cellular work (a catabolic reaction). • The product is now called ADP. • ATP can be rebuilt by using chemical energy released by the catabolism of glucose to rebuild the terminal phosphate energy rich bond (anabolism).