Notes #3
advertisement

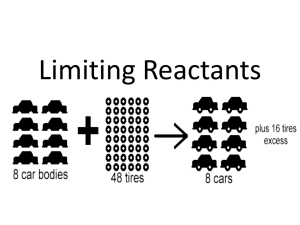
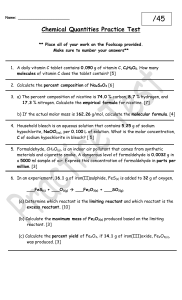
Chemistry Chapter 12 Notes #3 Limiting and Excess Reactions • What causes a chemical reaction to stop? • The reactants – or one of the reactantsget used up. • Limiting reactant – the reactant that gets used up first, therefore limiting (stopping) the reaction Example • How many sets of blue/red/yellow crayons can you make with the following? – 10 red crayons – 12 blue crayons – 6 yellow crayons • What is the limiting reactant? • Which were in excess (those not used up)? Example Hydrogen + Oxygen 4 Water molecules get created and an oxygen molecule is left over (excess), which means that Hydrogen is the limiting reactant The Math! • CsF + XeF6 -> CsXeF7 • How many moles of cesium xenon heptafluoride can be produced from the reaction of 12.5 mol cesium fluoride with 10.0 mol of xenon hexafluoride? • Hint – notice the 1:1:1 ratio • 10 mol CsXeF7 • You can only make as much as your least amount ! The Math • 6Na + Fe2O3 -> 3Na2O + 2Fe • If 100.0 g Na and 100.0 g Fe2O3 are used, determine the limiting reactant • • • • • 1) reactants = grams to moles 2) Mole to Mole comparison (ratio) 3) Limiting reactant becomes “given” 4) Go to Stoichiometry step #3 Excess reactant: Start Mass – Mass used The Math • 6Na + Fe2O3 -> 3Na2O + 2Fe • If 100.0 g Na and 100.0 g Fe2O3 are used, determine the limiting reactant The Math • 6Na + Fe2O3 -> 3Na2O + 2Fe • What is the mass of solid iron produced? The Math • 6Na + Fe2O3 -> 3Na2O + 2Fe • What is the mass of the excess reactant after the reaction? Percent Yield • If the conditions aren’t right, often a reaction will stop before the reactants are used up. • Percent yield shows the efficiency of the reaction. (compare to a letter grade on a test) • Actual yield (experiment) x 100 • Theoretical yield (math) Percent Yield • How might a business use percent yield in production for overall cost effectiveness? • Overall, you want percent yield to be as close to 100% as possible – Might result in changing conditions – Or more excess (which speeds up the reaction or help make it go through to completion) The Math • 6Na + Fe2O3 -> 3Na2O + 2Fe • If we ran this experiment and produced 62.0 g of Fe, what would the percent yield be?