Summary
advertisement

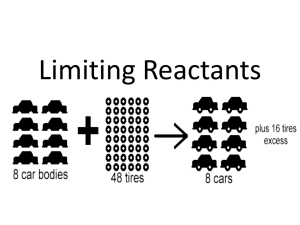
Summary of Unit 1: Stoichiometry and Gases Stoichiometry Gases Use the empirical formula to find molecular formulas with molar masses. There are two methods to find the limiting reactant: o Use reactant A and convert it into reactant B to see how much of reactant B is needed using stoichiometry. In this method, if the amount of reactant B needed is less than the amount of reactant B that you have, then reactant B is excess and reactant A is the limiting reactant. o Use both reactants A and B to find out how much product is produced. If reactant A produces less product, it is the limiting reactant and reactant B is in excess. “Once the limiting reactant is determined, ALL stoichiometry involving REACTANTS USED and PRODUCTS MADE should be based on the amount of LIMITING REACTANT.” (Stoichiometry notes, Hinton) The theoretical yield of an equation is the amount of product that is supposed to form according to calculations while the actual yield is the actual amount of product that is produced. actual yield The percent yield is the relation between the two using the equation: % Yield = x 100% theoretical yield In a combustion analysis, use the masses given to find the mass of all the elements in a compound. Then using the mass of the compound, find the percent composition and solve as shown in above. A hydrate is a stoichiometrically consistent amount of water in a compound. o XY3 nH2O XY3 + nH2O (g) (heat) The volume of a fixed quantity of gas maintained at a constant temperature is inversely proportional to the pressure. (Boyle’s Law) The volume of a fixed amount of gas maintained at a constant pressure is directly proportional to its absolute temperature. (Charles’ Law) Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. (Avogadro’s Hypothesis) The volume of a gas maintained at a constant temperature and pressure is directly proportional to the number of moles of the gas. (Avogadro’s Law) PV=nRT is the ideal gas law equation P𝑀 d= RT o Molar mass and pressure have a direct relationship with density and temperature is inversely related. STP is 0°C and 1.00 atm, 1.00 mole of gas occupies 22.4 L o Make sure that you only use 22.4 L/mol on gases 𝑃 𝑉 𝑃 𝑉 Use 1 1 = 2 2 when some of the values are held constant but others change 𝑛1 𝑇1 𝑛2 𝑇2 Constant n is sealed, constant V is a ridged container, constant T is insulated, and constant P is flexible The partial pressure of a gas can be related to its mass using P1 = X1Ptotal Kinetic molecular theory o Gases consist of large numbers of molecules (or atoms) in continuous, random motion. o These molecules have negligible volume compared to the total volume the gas occupies. o There are negligible attractive or repulsive forces between these molecules. o At constant temperature, the average kinetic energy of these molecules remains constant over time. (There is no net loss of kinetic energy due to their collisions, which are perfectly elastic.) o The molecules’ average kinetic energy is proportional to their absolute temperature. (Equipartition of energy.) 3𝑅𝑇 Use urms = √ 𝑟1 o 𝑟2 =√ 𝑀 to calculate the speed of a gas mole A less massive molecule will have a higher rms speed 𝑀2 𝑀1 (Graham’s law of effusion) Effusion: gas molecules escape into a vacuum Diffusion: gas molecules mix into another gas 𝑛 [P + a( )[V-bn] = nRT 𝑣 o It takes into account the attractive forces of the gas molecules and the molecular volume EQUATIONS urms = √ 𝑀 o urms: root mean squared speed o M: molar mass (must be in kg/mol) o R: 8.314 J/molK (given) o T: temperature (in Kelvin) o Gives answer in m/s P1 = X1Ptotal o P1: partial pressure o X1: mole fraction, na/ntotal o Ptotal: total pressure PV=nRT o P: pressure (1.000 atm = 101.325 kPa = 101 325 Pa = 14.69 psi = 760.0 Torr) o V: volume of Container o N: number of Moles 𝐿∙𝑎𝑡𝑚 𝐽 o R: 0.08206 = 8.314 (given) 𝑚𝑜𝑙∙𝐾 𝑚𝑜𝑙∙𝐾 o T: absolute Temperature (K) 𝑛 [P + a( )[V-bn] = nRT 𝑣 o P: real gas pressure o a: Van der Waals constant (given) o V: real container volume o b: Van der Waals constant (given) o n: number of moles 𝐿∙𝑎𝑡𝑚 𝐽 o R: 0.08206 = 8.314 (given) 𝑚𝑜𝑙∙𝐾 𝑚𝑜𝑙∙𝐾 o T: temperature (K) 𝑟1 𝑟2 =√ 𝑀2 𝑀1 o o 3𝑅𝑇 d= r: rate of effusion M: molar mass P𝑀 RT o o o o o d: density of the gas (g/L) P: pressure (L) M: molar mass 𝐿∙𝑎𝑡𝑚 𝐽 R: 0.08206 = 8.314 (given) 𝑚𝑜𝑙∙𝐾 𝑚𝑜𝑙∙𝐾 T: temperature (K)