Measuring rate of reaction
advertisement

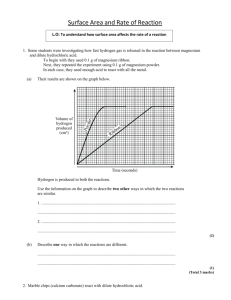
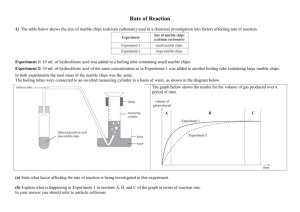
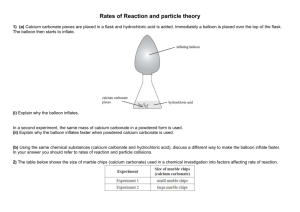
Rates of Reaction and Collision Theory Objectives: • To explain factors that affect the rate of chemical reactions. • To explain the collision theory and what happens to cause a chemical reaction. • To carry out practicals and calculate the rate of reactions. Chemical Reactions A reaction occurs when particles collide with each other. We can tell that a reaction has occurred by looking for the following: • a change in mass • a colour change • energy released (exothermic reaction) • bubbles or a gas being released • a new compound produced Rates of reaction Chemical reactions occur when particles of reactant collide with enough energy to react. Rates of Reaction Chemical reactions occur when different atoms or molecules collide: For the reaction to happen the particles must have a certain amount of energy – this is called the ACTIVATION ENERGY. The rate (speed) of a reaction happens depends on four things: 1) The temperature of the reactants, 2) Their concentration 3) Their surface area 4) Whether or not a catalyst is used Activation energy Chemical reactions involve the formation of bonds between atoms but often before new bonds can be Activation formed oldEnergy ones have is to be broken. energy given out as needed to new bonds This means that there has to be enough energy break form (activation energy) to start breakingexisting the old bonds bonds before a reaction can occur. Reactants Old bonds start to break New bonds form Changing the rate of a reaction.. 4 factors that affect the rate of reaction are: • Surface area • Concentration • Temperature • Catalyst Chemical reactions can be speeded up by increasing the temperature, surface area or the concentration of the the reactants. Catalysts also speed up chemical reactions. However at the end of a reaction the catalyst has not chemically changed, it just speeds up the reaction. Surface area The reactions of solids can clearly only take place at the surface of the solid. If we break a solid into smaller pieces we get more area and a faster reaction. Molecules collide with the surface of the solid Extra surface for molecules to collide with. Surface area If we grind up a solid to a powder we massively increase the surface area. We therefore massively increase the rate of any reaction Slow Very fast Concentration Reactions in solution involve dissolved particles that must collide before reaction is possible. The more crowded (concentrated) the solution, the faster the reaction. Collisions infrequent Collisions frequent Temperature At higher temperatures molecules move faster. As a result there are more collisions per second and so a faster reaction occurs. Slow molecules are also less likely to lead to a reaction than fast ones. More collisions per second Fewer collisions per second Catalysts A catalyst is a substance that speeds up a reaction without getting used up in the process. For example: In the present of Nickel catalyst, vegetable oil and hydrogen bond together to make margarine !! • Catalysts are used over and over again, they may need cleaning but are never used up. • Different reactions need different catalysts. Measuring the rate of reaction? There are several ways to measure the rate of reaction, these include: • collect the amount of gas released over a period of time. • record the colour change or precipitate produced over a period of time. • record a change in mass over a period of time So, you have to measure the amount of reactant used up or the amount of product produced per unit of time. Measuring rate of reaction Two common ways: 1) Measure how fast the products are formed 2) Measure how fast the reactants are used up Amount of product formed Rate of reaction graph Fast rate of reaction here Slower rate of reaction here due to reactants being used up Slower reaction Time Rates and Graphs Amount of product These show the increasing amount of product or the decreasing amount of reactant. Shallow gradient Slow reaction Steep gradient Fast reaction Time Acid and metal Reactive metals (e.g. Magnesium) react with acid to make hydrogen gas. magnesium + hydrochloric acid Mg(s) magnesium chloride + 2HCl(aq) MgCl2(aq) + hydrogen + H2(g) As the gas given off leaves the flask the total mass of the flask and its contents decreases slightly. Readings of the mass (g) can be taken. Usually at 1 minute intervals. HCl Mg 11.71 11.72 11.73 11.74 11.77 11.80 Acid and metal 1. Measure the agreed volume of acid / water into the conical flask. Cotton wool HCl 2. Have a loose plug of cotton wool to prevent “spitting” of droplets of liquid. 3. Have a piece of magnesium of known mass ready. 4. Add the magnesium, place the cotton wool in the neck and start taking mass readings immediately. Mg 11.71 11.72 11.73 11.74 11.77 11.80 Time (s) Reading (cm3) 0 0 60 120 Acid and marble Marble chips are calcium carbonate. They react with acid to make a gas (carbon dioxide) calcium + hydrochloric carbonate acid calcium + water chloride + carbon dioxide CaCO3(s) + 2HCl(aq) CaCl2(aq) + H2O(l) + CO2(g) Glass tube The gas given off can be collected in a syringe and readings taken every 30 seconds or so. Gas syringe Hydrochloric acid Marble chips Acid and marble 1. Measure the agreed mass of marble chips. 2. Set up the syringe, flask and connector 3. Measure the acid /water. 4. Add the marble chips and quickly insert the bung and start stop clock. 5. Take syringe readings at 30 second intervals. Time (s) 0 Reading (cm3) 0 Science Coursework Investigating how concentration affects the rate of reaction between Hydrochloric Acid and Marble Chips. PLANNING Aim – To find out how changing the concentration of acid affects the rate of reaction with Calcium Carbonate. Preliminary Work – Write up any extra work you have done that has helped you plan your work. You must include a results table and graph to show how it helped your plan. HINT: The data collected needs to be spread out to allow you to spot patterns. Reactions that are too quick will be no good. Preliminary work Volume of gas released (cm3) Time (s) 0.5mol T1 T2 1.0mol T1 T2 1.5mol T1 T2 2.0mol T1 T2 0 20 40 60 80 100 120 Plot a graph, decide which concentrations you can use to make a range and be able to explain why ... Volume of Gas (ml) PLOTTING YOUR GRAPH Time (seconds) Apparatus and Diagram • conical flask • 25ml of Hydrochloric Acid (quote what concentrations you will use) • goggles • delivery tube • tub of water • bung • 2 or 3g small marble chips YOU DECIDE • measuring cylinder • digital balance • stop watch The important parts of your “Strategy” planning marks are now for justifying and explaining your choice of apparatus. Make any relevant comments here about how your preliminary work helped you make any decisions. Fair Test There are 4 factors that can affect the rate of reaction. These are : 1) You must explain how and why 2) you are controlling 3 of these 3) factors. Detailed explanations required!! 4) In this investigation I am only going to change the ____________________. I must control the other factors. I will do this by using the __________ marble chips and using the same equipment for each test. I will also ________________ and work out the averages. Safety – How will you make sure the work is done safely ? I have to make sure that I work safely, I will ......... Method Write a fully detailed list of instructions. You must include: • Instructions how to do the work • How long each test will last for • How often will you take your results • What concentrations of acid to use and how many repeats to do RESULTS Test 1 with 0.2m Hydrochloric Acid Volume of gas released (ml) Time (s) 0 20 40 60 80 100 Test 1 Test 2 Test 3 Average RESULTS Test 2 with 0.4m Hydrochloric Acid Volume of gas released (ml) Time (s) 0 20 40 60 80 100 Test 1 Test 2 Test 3 Average RESULTS Test 3 with 0.6m Hydrochloric Acid Volume of gas released (ml) Time (s) 0 20 40 60 80 100 Test 1 Test 2 Test 3 Average RESULTS Test 4 with 0.8m Hydrochloric Acid Volume of gas released (ml) Time (s) 0 20 40 60 80 100 Test 1 Test 2 Test 3 Average RESULTS Test 5 with 1.0m Hydrochloric Acid Volume of gas released (ml) Time (s) 0 20 40 60 80 100 Test 1 Test 2 Test 3 Average AVERAGE RESULTS Volume of gas released (ml) Time (s) 0 20 40 60 80 100 0.2m 0.4m 0.6m 0.8m 1.0m Volume of Gas (ml) PLOTTING YOUR GRAPH Time (seconds) ANALYSIS By looking at my graph I can see that as concentration increased the line got steeper. This means the reaction was happening quicker. The quickest reaction was with the 1.25m acid, this had the steepest line. The concentrated acid reacted the quickest because it has more acid particles in the same volume. This meant there were more successful collisions with the marble chips in the flask. Acid Particles Marble Chips I can use my graph to work out the rate of reaction for each acid I used. Rate of reaction = Amount of gas (ml/s) (ml) Time taken (s) Work out the rate of reaction for each concentration of acid for the first 60 seconds. Show your working out. Is there any relationship between your results? Explain if your results support the collision theory, if you double the concentration does the rate of reaction soluble too ? Was your prediction correct ? Explain what you said and why it is right or wrong. EVALUATION In this work I have found out that increasing the concentration of the acid increases the rate of a reaction. On my graph there are _______ anomalous incorrect results. I have circled them and not included them in line of best fit. My results can be made more reliable by finding a way of controlling the surface area of the marble chips. Because they were not exactly the same the test was not 100% fair and accurate. I also need to do all my results in the same lesson or day to ensure that the working room temperature is the same.