questions on rates of reaction (1)
advertisement

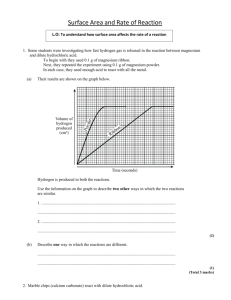
Worksheet G5.1 QUESTIONS ON RATES OF REACTION (1) (Particle Size and Concentration of Solutions) 1) Calcium carbonate reacts with dilute nitric acid to give off carbon dioxide gas:calcium + nitric calcium + water + carbon carbonate acid nitrate dioxide CaCO3(s) + 2HNO3(aq) Ca(NO3)2(aq) + H2O(l) + CO2(g) 40cm3 of 2M nitric acid and about 20g (a large excess) of marble chips are reacted in a conical flask placed on a digital balance. The mass is recorded at regular times. The CO2 escapes from the flask, so the mass decreases with time. The loss in mass is equal to the mass of CO2 formed. Two experiments are carried out. In experiment A 20g of small marble chips are used, while in experiment B 20g of large marble chips are used. The results are given in the table below:Time (s) A : Mass Loss (g) B : Mass Loss (g) 30 60 90 120 180 240 300 360 420 480 540 600 0.45 0.85 1.13 1.31 1.48 1.54 1.56 1.58 1.59 1.60 1.60 1.60 0.18 0.38 0.57 0.75 1.05 1.25 1.38 1.47 1.53 1.57 1.59 1.60 a) Draw a set of axes with “Mass Loss” on the y-axis (vertical) and “Time” on the x-axis. Plot BOTH sets of results on the same axis. Draw smooth curves of best fit for each set of points and label them A and B. (5) b) Look at the two curves over the first 60 seconds. i) Which experiment (A or B) had the greater initial rate? …….…A………. (1) ii) Explain your reasoning. A lost 0.85g in the first 60s (0.85g/60s = 0.014g/s) and B only lost 0.38g (0.38g/60s = 0.0063g/s). A lost more mass in the same amount of time, so it had the faster initial rate. (1) c) How can you tell from the graph when the reaction stopped in each experiment? The graph stopped increasing (the gradient became 0, or flat) (1) d) The mass of marble in each experiment was the same (20g). How did the size of the marble chips affect the rate of this reaction? The larger the size of the marble chip, the slower the rate. 1 (1) Worksheet G5.1 e) Explain your answer to part (d) in terms of the surface area of the marble chips. In Experiment A, the marble chips were smaller, so they had more surface area (b/c there were more of them, since there were 20g worth in both experiments. With more surface area, there is more area for collisions, so we will have more collisions, which means a greater probability of effective collisions (proper orientation and energy), which means a faster rate.(2) f) Sketch on your graph from part (a) the curve you would expect if the experiment were repeated with the same volume of 2M nitric acid and 20g of powdered marble. (2) It would happen faster, so the line would reach 1.6 faster than both A & B. [TOTAL = 13] 2) Magnesium reacts with dilute hydrochloric acid and gives off hydrogen gas. 5cm lengths of Mg ribbon are added to excess hydrochloric acid and the hydrogen is collected in a gas syringe. The time taken to collect 10cm3 of gas is measured for different concentrations of the acid. The results are shown in the table below:Concentration of acid (mol.dm-3) Time to collect 10cm3 hydrogen (s) 0.2 60 0.4 30 0.6 20 0.8 15 1.0 12 a) Plot a graph of “Time” on the y-axis (vertical) against “Concentration” on the x-axis and draw a smooth curve through the points. (5) b) What effect does increasing the acid concentration have on the time taken to produce 10cm3 of hydrogen gas? The higher the acid concentration, the less time taken to produce 10cm3 of H2 gas(1) c) Use your graph to find out how long it would take to collect 10cm3 of hydrogen for an acid concentration of 0.5mol.dm-3. (Show clearly how you got your answer.) ~25seconds (2) d) The reaction is believed to occur as a result of collisions between particles of acid in the solution and Mg atoms on the surface of the metal. Use diagrams to help you explain carefully in terms of collision theory the effect of increasing the acid concentration on the rate of the reaction. Increasing the concentration means more particles are available – this means more collisions. More collisions means a higher probability of effective collisions (particles colliding with the proper orientation and energy) – which means a faster reaction rate. (4) 2 Worksheet G5.1 e) What is the effect on the rate of the reaction of using magnesium powder instead of magnesium ribbon? Explain this clearly, again using collision theory. Powder – more surface area. See above (1e) for explanation (3) [TOTAL = 15] 3) A compound called benzene diazonium chloride can only exist in solution in water below 10OC. At room temperature it decomposes and gives off nitrogen gas. A student decided to investigate how the rate of the decomposition reaction varied with the concentration of the solution. He made up several different concentrations of solution and kept them in the fridge until he needed them. He decided to time how long it took for each solution to produce 20cm3 of nitrogen gas. He put 100cm3 of each solution in a conical flask in a water bath at 20OC and collected the gas in a gas syringe. His results are given below:Concentration of solution (mol.dm-3) 0.05 0.10 0.15 0.20 Time to collect 20cm3 N2 gas (s) 715 370 250 185 0.25 0.30 150 125 Reaction Rate = 1/time (s-1) 0.0000699 0.000270 0.0006 0.00108 0. 0.00167 0.0024 1000 Reaction Rate 0.0699 0.27 0.27 0.6 1.08 1.67 2.4 a) Draw a labelled diagram of the apparatus the student could have used. (3) b) Complete the two blank columns in the table (the last one is to give you values which are easier to plot!). (2) c) Plot a graph of “1000 Reaction Rate” on the y-axis (vertical) against “Concentration” on the x-axis. Make sure you start both axes at zero and label them. (4) d) Draw a line of best fit through the points. (1) e) Describe as carefully as you can what the graph tells you about how the reaction rate changes with the concentration of the solution. The higher the concentration, the faster the rate (2) [TOTAL = 12] TOTAL MARKS FOR WORKSHEET = 40 3