surface area
advertisement

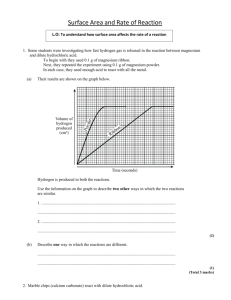
Experiment guide Surface Area ../Chemistry/25_Guide-4.wav Objectives: 1) To investigate if and how the surface area of reactants can affect the rate of reaction 2) To discover how we can measure the rate of a reaction Equipment Diluted hydrochloric acid small marble chips of different sizes and powdered chalk 3 Erlenmeyer flasks 3 watch glasses 1 cork for the flask in which it is installed a tubing glass connected to a rubber tubing an apparatus made of water bath and a upside-down graduated cylinder full of water and supported by a stand a watch Caution Gloves and goggles must be worn Procedure: follow the steps and do the exercises 1- Prepare 3 flasks with 40ml of hydrochloric acid (1M) 2- Prepare 3 samples of the same mass (1 – 2 g) of: a) small marble chips b) bigger marble chips c) powdered chalk (Use the three watch glasses to measure the mass of the three samples) 3- Set up the apparatus as shown (see picture). Arrange the three samples according to their size in increasing order. 4- Pour the first samples in the hydrochloric acid of the first flask; immediately put the bung into the flask and start timing. Be careful! HCl can squirt! 5- Every 15 seconds (for few minutes) record in the “data and observation table” the volume of gas produced by the reaction, gathered at the top of the graduated cylinder, in correspondence of the relative time 6- Repeat steps 4 and 5 using the other samples Experimental data Time (s) O 15 30 Sample one Volume (ml) Sample two Volume (ml) Sample tree Volume (ml) Data analysis and findings I ) Graph the data of each reaction: a) plot gas volume on the Y axis and the time on the X axis b) draw a line that best fits the data points II) Do the following exercises, to understand the objectives of this experiment EXSERCISES 1- Think of having a plastiline cube and cutting it. How does the surface area change cutting the solid? It increases It decreases It remains constant Referring to the experiment you did: 2Which are the reactants? Which are the products? H2O(l) CO2(g) CaCO3(s) HCl(aq) CaCl2(aq) 3- What are the names of these substances? H2O………………………………… CaCO3 ……………………………… CaCl2 …………………………………. CO2………………………………………….. HCl ……………………………………… 4- Write the chemical equation ……….….(s) + 2……....….(aq). ..............(l) + ….…...….(aq) + .............. (g) 5- What tells you that a reaction has occurred? Something changed colour The formation of bubbles in the solution The formation of a precipitate The solution became cloudy because of Forming of solid Forming of liquid Forming of gas 6- Which among these products is responsible for the observed phenomenon? H2O(l) CO2(g) CaCO3(s) HCl(aq) CaCl2(aq) 7- These graphs show the amount of gas produced as time goes by, in similar reactions. The two reactions occurred in different conditions. Amount of gas produced (ml) 1 2 -When the lines become level (no more gas is produced) the two reactions Time (s) have a higher rate are finished none of the two - At the end of the two reactions the two lines show the same amount of product, so we can say that, at the start, the amount of reactants was the same different none of the two - The two lines are both steepest at the start, but number 1 is steeper, which means that reaction 1 finishes first and its rate is slower than reaction 2 its rate is faster than reaction 2 8- Which reaction was faster? The reaction with small marble chips The reaction with bigger marble chips The reaction with powdered chalk 9- What was the difference in the three observed systems of reaction? Presence of catalyst Surface area of reactants Reactants concentration Pressure Nature of reactants Stirring Temperature 10- What was the first objective of this experiment? To investigate what can affect the rate of a reaction To discover how we can measure the rate of a reaction To give a definition of the rate of reaction To investigate if and how the surface area of a solid reactant can affect the rate of a reaction 11- How did we establish that the reaction with powdered chalk was faster? measuring the amount of gas produced in given times measuring the mass loss due to the formation of gas in a given time measuring how much a certain reactant is used up in a given time 12- How can the rate of a reaction be measured? determining how quickly one of the reactants is consumed determining how quickly one of the products is made up both of the two 13- What was the second objective of our experiment? To investigate what can affect the rate of a reaction To discover how we can measure the rate of a reaction To investigate if and haw the surface area of a solid reactant can affect the rate of reaction GUIDED REPORT - In the reaction that we observed the reactants were - calcium carbonate (CaCO3) that is a solid compound and - hydrochloric acid (HCl) that is in solution. The products of this reaction were calcium chloride (CaCl2) that is an ionic compound dissolved in water, carbon dioxide (CO2) that is a gas that escapes from the solution and water. We can describe the reaction we observed with the following chemical symbology ……….….(s) + 2……....….(aq). ..............(l) + ….…...….(aq) + .............. (g) - We studied the rate of the reaction measuring how much ………… was produced in a certain ………… (15 seconds, 30 seconds, ...) We repeated the ………… reaction three times, but each time we changed the size of the marble chips and, therefore, the ………… ………… . of the solid reactant. (We used bigger chips, smaller chips and powdered chalk) - We discovered that the same chemical reaction can occur with several different ………… depending on the ………… ………… of reactants; in fact the more the surface area of reactants the more the ………… of reaction. - The graph shows the amount of gas formed in relation with the time. Amount of gas formed (ml) Graphs 1, 2 and 3 represent the reaction taking place with the same initial amounts. Time (s) The initial steepness of the line represents the ………… of the reaction. The steeper the initial slope is the ………… the reaction, in fact the reaction ends more quickly. Graph number 1 represents the reaction of ………… chalk, graph number 2 represents the reaction of ………… marble chips, graph number 3 represents the reaction of ………… marble chips.