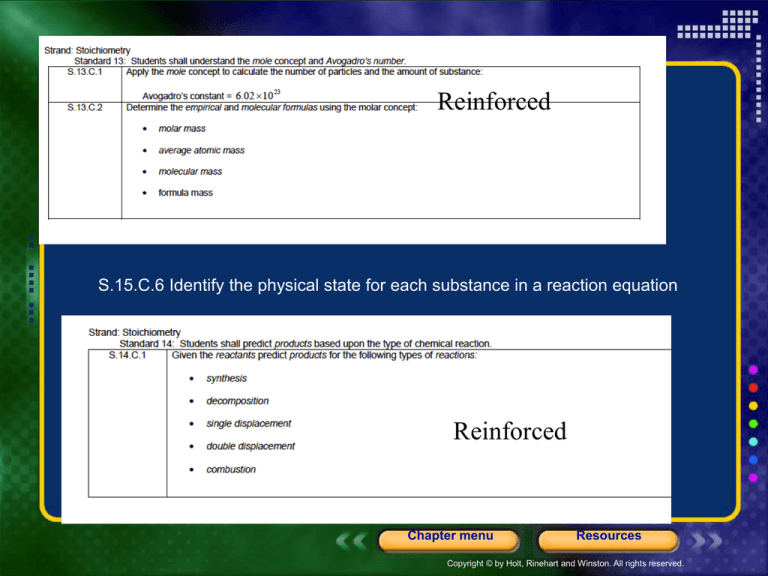
Reinforced
S.15.C.6 Identify the physical state for each substance in a reaction equation
Reinforced
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Main Standards
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Stoichiometry
Table of Contents
Section 2 Ideal Stoichiometric Calculations
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Objective
• Calculate the amount in moles of a reactant or a
product from the amount in moles of a different
reactant or product.
• Calculate the mass of a reactant or a product from the
amount in moles of a different reactant or product.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Objectives, continued
• Calculate the amount in moles of a reactant or a
product from the mass of a different reactant or
product.
• Calculate the mass of a reactant or a product from the
mass of a different reactant or product.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Quantities in Moles
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Visual Concepts
Conversion of Quantities in Moles
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_003036809x/
Visual Concept
student/ch09/sec02/vc00/hc609_02_v00
fs.htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Solving
Mass-Mass
Stoichiometry
Problems
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Quantities in Moles, continued
Sample Problem A
In a spacecraft, the carbon dioxide exhaled by astronauts
can be removed by its reaction with lithium hydroxide,
LiOH, according to the following balanced chemical
equation.
CO2(g) + 2LiOH(s) Li2CO3(s) + H2O(l)
How many moles of lithium hydroxide are required to react
with 20 mol CO2, the average amount exhaled by a
person each day?
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Quantities in Moles, continued
Sample Problem A Solution
CO2(g) + 2LiOH(s) Li2CO3(s) + H2O(l)
Given: amount of CO2 = 20 mol
Unknown: amount of LiOH (mol)
Solution:
mol ratio
mol LiOH
mol CO2
mol LiOH
mol CO2
2 mol LiOH
20 mol CO2
40 mol LiOH
1 mol CO2
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Practice problems pg. 306
• Write the balanced equation for practice problems pg.
306 and answer the question in your notes.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
ANSWERS
Practice problems pg. 306
• Write the balanced equation for practice problems pg.
306 and answer the question in your notes.
Make sure you understand why.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Amounts in Moles to Mass
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Solving Stoichiometry
Problems with Moles
or Grams
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Amounts in Moles to Mass,
continued
Sample Problem B
In photosynthesis, plants use energy from the sun to
produce glucose, C6H12O6, and oxygen from the
reaction of carbon dioxide and water.
What mass, in grams, of glucose is produced when
3.00 mol of water react with carbon dioxide?
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Amounts in Moles to Mass, continued
Sample Problem B Solution
Given: amount of H2O = 3.00 mol
Unknown: mass of C6H12O6 produced (g)
Solution:
Balanced Equation: 6CO2(g) + 6H2O(l) C6H12O6(s) + 6O2(g)
mol ratio
mol H2O
3.00 mol H2O
mol C6H12O6
mol H2O
1 mol C6H12O6
6 mol H2O
molar mass factor
g C6H12O6
mol C6H12O6
g C6H12O6
180.18 g C6H12O6
1 mol C6H12O6
=
90.1 g C6H12O6
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Practice problems pg. 308
• Write the balanced equation for practice problems pg.
308 #1-2 and answer the question in your notes.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
ANSWERS
Practice problems pg. 308
• Write the balanced equation for practice problems pg. 308
#1-2 and answer the question in your notes.
Make sure you understand why.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Mass to Amounts in Moles
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Visual Concepts
Mass and Number of Moles of an Unknown
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_00303680
Visual Concept
9x/student/ch09/sec02/vc01/hc609_0
2_v01fs.htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Mass to Amounts in Moles,
continued
Sample Problem D
The first step in the industrial manufacture of nitric acid
is the catalytic oxidation of ammonia.
NH3(g) + O2(g) NO(g) + H2O(g) (unbalanced)
The reaction is run using 824 g NH3 and excess
oxygen.
a. How many moles of NO are formed?
b. How many moles of H2O are formed?
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Conversions of Mass to Amounts in Moles, continued
Sample Problem D Solution
Given: mass of NH3 = 824 g
Unknown: a. amount of NO produced (mol)
b. amount of H2O produced (mol)
Solution:
Balanced Equation: 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g)
molar mass factor
a.
b.
g NH3
mol NH3
g NH3
mol NH3
g NH3
g NH3
mol ratio
mol NO
mol NH3
mol NO
mol H2O
mol H2O
mol NH3
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Section 2 Ideal Stoichiometric
Calculations
Chapter 9
Conversions of Mass to Amounts in Moles,
continued
Sample Problem D Solution, continued
molar mass factor
a. 824 g NH3
b. 824 g NH3
1 mol NH3
17.04 g NH3
1 mol NH3
17.04 g NH3
mol ratio
4 mol NO
48.4 mol NO
4 mol NH3
6 mol H2O
4 mol NH3
Chapter menu
72.5 mol H2O
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Practice problems pg. 309
• Write the balanced equation for practice problems pg. 309
#1-2 and answer the question in your notes.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
ANSWERS
Practice problems pg. 309
• Write the balanced equation for practice problems pg. 309
#1-2 and answer the question in your notes.
Make sure you understand why.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Mass-Mass to Calculations
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Visual Concepts
Mass-Mass Calculations
Click below to watch the Visual Concept.
http://my.hrw.com/sh/hc6_003036809x/stu
Visual Concept
dent/ch09/sec02/vc02/hc609_02_v02fs.htm
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Solving Mass-Mass Problems
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Mass-Mass to Calculations, continued
Sample Problem E
Tin(II) fluoride, SnF2, is used in some toothpastes. It is
made by the reaction of tin with hydrogen fluoride
according to the following equation.
Sn(s) + 2HF(g) SnF2(s) + H2(g)
How many grams of SnF2 are produced from the
reaction of 30.00 g HF with Sn?
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Mass-Mass to Calculations, continued
Sample Problem E Solution
Given: amount of HF = 30.00 g
Unknown: mass of SnF2 produced (g)
Solution:
molar mass factor
mol ratio
molar mass factor
mol SnF2
g SnF2
mol HF
g HF
g SnF2
g HF
mol HF
mol SnF2
1 mol SnF2
156.71 g SnF2
1 mol HF
g HF
20.01 g HF
2 mol HF
1 mol SnF2
= 117.5 g SnF2
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Practice Problems p.311 #1-3
Chapter 9
• Write a balanced equation before answering
questions.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Practice Problems p.311 #1-3 ANSWERS
Chapter 9
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Solving Various Types of Stoichiometry Problems
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Solving Various Types of Stoichiometry Problems
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Solving Volume-Volume
Problems
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Section 2 Ideal Stoichiometric
Calculations
Solving Particle Problems
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 9
Visual Concepts
Ideal Stoichiometric Calculations
Click below to watch the PROBLEM ACTIVITIES
CALCULATING MOLES REACTED
MASS TO MOLES PROBLEM
MOLES TO MASS PROBLEM
MOLES TO MASS #2
MASS TO MASS PROBLEM
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Online Self-Check Quiz
Complete the online Quiz and record answers.
Ask if you have any questions about your
answers.
click here for online Quiz 9.2
(7 questions)
You must be in the “Play mode” for the
slideshow for hyperlink to work.
Slide
of 25
© Copyright Pearson Prentice Hall
End Show
VIDEOS FOR ADDITIONAL INSTRUCTION
Additional Videos for
Section 9.2: Ideal Stoichiometric Calculations
None at this time
Slide
of 28
© Copyright Pearson Prentice Hall
End Show
End of Chapter 9.2 Show
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.









