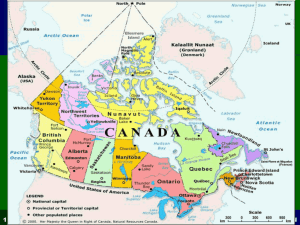
Chapter L2
Section 3 Types of Chemical Reactions
Bellringer
Explain what happens to bonds when a chemical
reaction occurs? What are the signs that a
chemical reaction occurred?
Write your responses in your science journal.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
MS-PS1-2
Physical and Chemical Properties
Objective
• Interpret and write simple chemical formulas.
• Describe four types of chemical reactions.
• Classify a chemical equation as one of four types of
chemical reactions.
Vocabulary
Agenda
Chemical formula
Today we will:
Chemical equation
• Complete the do now
Reactant
• Watch a video and complete
product
quiz on chemical equations.
• Guided instruction/ teamwork
practice.
• Review and exit ticket. Homework
worksheet
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter L2
Section 2 Chemical Formulas and Equations
Chemical Formulas
• A chemical formula is a combination of chemical
symbols and numbers to represent a substance. A
chemical formula shows how many atoms of each
kind are present in a molecule.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter L2
Section 2 Chemical Formulas and Equations
Chemical Equations
• Describing Reactions by Using Equations A
chemical equation uses chemical symbols and
formulas as a shortcut to describe a chemical reaction.
• From Reactants to Products The starting materials
in a reaction are reactants. The substances formed
from a reaction are products.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter L2
Section 2 Chemical Formulas and Equations
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter L2
Section 2 Chemical Formulas and Equations
Chemical Equations, continued
• The Importance of Accuracy The symbol or formula
for each substance in a chemical equation must be
written correctly or it will not correctly describe the
reaction. Some formulas and symbols can be confused.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter L2
Section 3 Types of Chemical Reactions
Synthesis Reactions
• A synthesis reaction is a reaction in which two or
more substances combine to form one new compound.
• For example, a synthesis reaction takes place when
sodium reacts with chlorine to produce sodium chloride,
which you know as table salt.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter L2
Section 3 Types of Chemical Reactions
Decomposition Reactions
• A decomposition reaction is a reaction in which a
single compound breaks down to form two or more
simpler substances.
• Decomposition is the reverse of synthesis.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter L2
Section 3 Types of Chemical Reactions
Single-Displacement Reactions
• A single-displacement reaction is a reaction in
which an element replaces another element that is part
of a compound. The products of single-displacement
reactions are a new compound and a different element.
• Reactivity of Elements In a single-displacement
reaction, a more reactive element can displace a less
reactive element in a compound.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter L2
Section 3 Types of Chemical Reactions
Double-Displacement Reactions
• A double-displacement reaction is a reaction in
which ions from two compounds exchange places.
One of the products of this type of reaction is often a
gas or a precipitate.
• The next slide shows models of each of the four
types of chemical reactions.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Exit Ticket
Describe and name the 4 types
of chemical reactions.
Chapter menu
Resources
Copyright © by Holt, Rinehart and Winston. All rights reserved.