Cell Physiology
advertisement

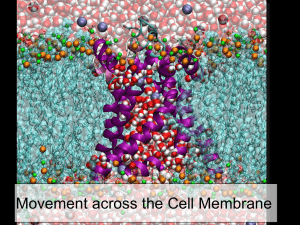
How things get into cells: Principles of diffusion, osmosis, and the nature of biological membranes. Diffusion Movement of substances from an area of high concentration to an area of low concentration Depends on 2nd law of thermodynamics Osmosis: special case of diffusion of water Biological membranes Lipids =barriers; proteins = channels. 1 Movement of molecules depends on: 1. Kinetic energy higher temperature = more kinetic energy 2. Concentration gradient – more of something in one area than another Second Law of Thermodynamics: all things tend toward entropy. If there’s more of something in one area, it will spread out. 2 Diffusion Passive process Depends on concentration and kinetic energy Does not require energy Moves substances from an area of high concentration to an area of low concentration Down a concentration gradient 3 Osmosis: a special instance of diffusion The most concentrated form of water is pure water. To make water less concentrated, we dissolve substances in it. Concentration of one solution relative to another Isotonic – equal concentrations Hypertonic – more concentrated Hypotonic – less concentrated 4 Tonicity – relative concentrations of solutions Isotonic – two solutions contain the same amount of substance dissolved in themequal concentrations Hypertonic – a solution containing a greater amount of dissolved substance- more concentrated Hypotonic – a solution containing a lesser amount of dissolved substance – less concentrated Osmosis: The movement of water across a selectively permeable membrane, down a concentration gradient. 5 6 7 Osmosis: special case of the diffusion of water. Movement of water across a semi permeable membrane. If the environment is: Isotonic: No NET flow. Hypertonic: Water flows OUT of cell. Hypotonic: Water flows IN. Water flows from where it (the water) is in high concentration to where it is in low concentration. 8 Osmosis is an important phenomenon in biology Red blood cells must be collected carefully In a hypotonic solution (distilled water), water enters the RBCs and explodes them. The action of penicillin depends on it Bacteria are protected by a cell wall. When drugs damage the wall, water rushes in and explodes the bacterium, killing it. Filtering action of the kidney depends on it Water is drawn back out of urine. 9 The physical law that molecules travel down a concentration gradient (from a region of high to low concentration) drives most movement of molecules in and out of cells. Other movement of molecules and particles e.g., movement “against” a concentration gradient, requires some form of metabolic energy. 10 Entry of particles and molecules attached to the cell membrane Requires: •Direct access of cell membrane to outside •Cytoskeleton •Source of energy Thus NOT done by cells with cell walls (plants, fungi, bacteria) or cells without a cytoskeleton (bacteria). http://bio.winona.msus.edu/bates/genbio/images/endocytosis.gif 11 The type of molecule affects how it gets through a membrane Small molecules can pass through a membrane Water; Gases such as O2 and CO2 Lipid molecules can Dissolve in lipid bilayer, pass through membrane Many drugs, vitamins, hormones are lipid soluble Larger, hydrophilic molecules cannot Ions, sugars, amino acids cannot Transport proteins required 12 Transport through membranes Simple diffusion Molecules travel down concentration gradient Membrane is not a barrier to their passage Facilitated diffusion Molecules travel down concentration gradient Cannot pass through lipid bilayer; their passage is facilitated by protein transporters Active transport Molecules travel against concentration gradient Requires input of metabolic energy (ATP), transporter 13 How molecules get through the membrane http://www.rpi.edu/dept/chem-eng/Biotech-Environ/Membranes/bauerp/diff.gif 14 Active transport: movement of molecules against a concentration gradient. Requires Energy. 15 Transport into cells: a step in the middle of a process All cells need raw materials For maintaining cells, building new ones All cells need a source of energy For continuing cell functions like movement For building new molecules, new cells Chemical reactions that use or release energy: Metabolism 16 Food preparation, consumption, digestion (break down into smaller molecules.) Small molecules absorbed or transported into cells. http://www.nlm.nih.gov/medlineplus/ency/images/ency/fullsize/8710.jpg 17 Chemical reactions carried out by enzymes Once inside the cell, molecules are used as raw materials or for their energy. The chemical reactions involved are carried out by protein molecules called enzymes. Enzymes are the tools of the cell and have several important properties. 18 Enzymes are catalysts Catalysts speed up the rate of a chemical reaction 2H2 + O2 2H2O Reactions must occur quickly in a cell. A catalyst is not used up Enzymes are tools, so think “hammer” Used over and over to pound nails 19 Enzymes overcome “activation energy” Enzymes give an extra push to reactions that don’t require energy to finish. Enzymes are facilitators: they get all the reactants together on the enzyme’s surface so they can react. 20 Enzymes are specific There are thousands of different reactions that take place in a cell, most of them going on at the same time. To speed up each one, there is a different enzyme. Each type of enzyme can speed up only 1 type of chemical reaction. Enzymes are proteins, and their 3D shape is what makes them specific. Think “wrenches” Instructions for making enzymes thus found in the DNA 21 The next lecture(s) will discuss how cells take molecules, break them down and release the energy in them, and put them back together in the unique way required by the cells. 22