File
advertisement
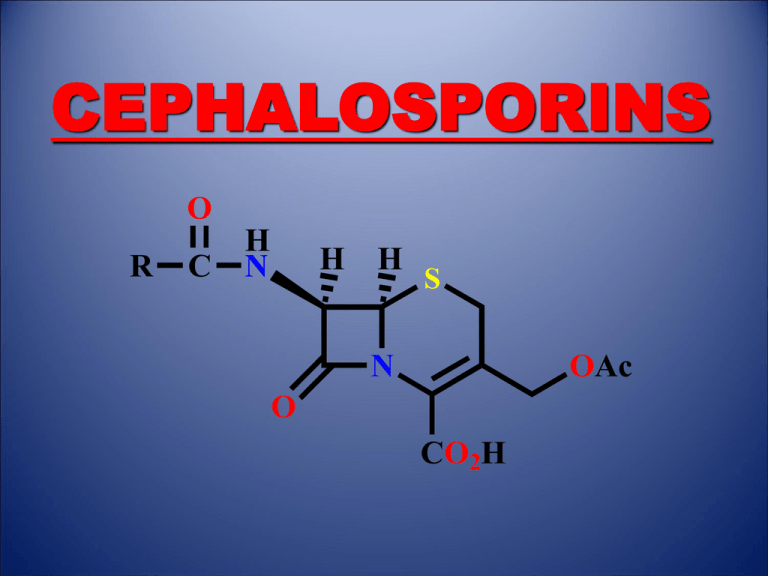
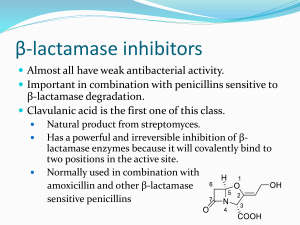
CEPHALOSPORINS O R C H N H H S N OAc O CO2H 1. Introduction •Antibacterial agents which inhibit bacterial cell wall synthesis •Discovered from a fungal colony in Sardinian sewer water (1948) •Cephalosporin C identified in 1961 2. Structure of Cephalosporin C 7-Aminoadipic side chain H H N H2N H CO2H 7 8 O H 6 5 N S 1 4 2 3 O O Dihydrothiazine ring b-Lactam ring H H Me O CO2H H2N C S N O O CO2H C Me O 7-Aminocephalosporinic acid (7-ACA) 3. Properties of Cephalosporin C H H N H2N H CO2H 7 8 O H 6 5 N S 1 4 2 3 O CO2H O C Me O Disadvantages •Polar due to the side chain - difficult to isolate and purify •Low potency - limited to the treatment of urinary tract infections where it is concentrated in the urine •Not absorbed orally Advantages •Non toxic •Lower risk of allergic reactions compared to penicillins •More stable to acid conditions •More stable to b-lactamases •Ratio of activity vs Gram -ve and Gram +ve bacteria is better Conclusion •Useful as a lead compound 4. Biosynthesis of Cephalosporins H2N R OH O H Cys SH HO CO2H Me Val Me H2N CO2H C O Me H H N R O H S N O O CO2H C O Me 5. SAR of Cephalosporins H H N R 7 8 O H 6 5 N S 1 4 2 3 O CO2H O C Me O •Similar to penicillins •The b-lactam ring is crucial to the mechanism •The carboxylic acid at position 4 is important to binding •The bicyclic system is important in increasing ring strain •Stereochemistry is important •The acetoxy substituent is important to the mechanism Possible modifications •7-Acylamino side chain •3-Acetoxymethyl side chain •Substitution at C-7 6. Mechanism of Action H H N 7 R O H S N O O CO2H C O Me H En z yme S -CH3CO2- O N O O Ser OH Ser H H N R En z ym e Note The acetoxy group acts as a good leaving group and aids the mechanism CO2H 7. Variation of the 7-Acylamino Side Chain •Not possible to generate analogues by fermentation •Not possible to generate analogues by a full synthesis •Restricted to semi-synthetic procedure H2N H H S RCOCl N O O CO2H H H N R C Me O H N O O O 7-ACA •7-ACA not available by fermentation •7-ACA not available by enzymatic hydrolysis of cephalosporin C •Generated by a chemical hydrolysis S CO2H C O Me 7. Variation of the 7-Acylamino Side Chain Generation of 7-ACA •Need to hydrolyse a relatively unreactive secondary amide in the presence of a labile blactam ring H N R1 H H S PCl5 7 O N 4 O 3 R1 N H R1 ROH Cl OAc H H2O OR O CO2SiMe3 N -R1CO2H O Im in o ch lori de Im in o e th e r Protecting group H2N H H N CO2H 7-AC A H H S R2COCl OAc O H N R2 S O N OAc O CO2H Range of cephalosporins 8. First Generation Cephalosporins Cephalothin H H N 7 S O H S 3 N OAc O CO2H •More active than penicillin G vs. some Gram -ve bacteria •Less likely to cause allergic reactions •Useful vs. penicillinase producing strains of S. aureus •Not active vs. Pseudonomas aeruginosa •Poorly absorbed from GIT •Administered by injection •Metabolised to give a free 3-hydroxymethyl group (deacetylation) •Metabolite is less active 8. First Generation Cephalosporins Cephalothin - drug metabolism H H N 7 S O H H H N S 3 N S OAc Metabolism O O H S N OH O CO2H CO2H •Less active •OH is a poorer leaving group Strategy Replace the acetoxy group with a metabolically stable leaving group 8. First Generation Cephalosporins Cephaloridine H H N 7 S O H S 3 N N O CO2 •The pyridine ring is stable to metabolism •The pyridine ring is a good leaving group (neutralisation of charge) •Exists as a zwitterion and is soluble in water •Poorly absorbed through the gut wall •Administered by injection 8. First Generation Cephalosporins Cefalexin H2N H H H N 7 O H S 3 N Me O CO2H •The 3-methyl group is a poor leaving group •Methyl group is bad for activity but aids oral absorption •Can be administered orally •A hydrophilic amino group at the a-carbon of the side chain helps to compensate for the loss of activity due to the methyl group 8. First Generation Cephalosporins Synthesis of cephalosporins with a 3-methyl substituent R C H N O H 6 N O OH O S 2 S H2O2 Me Me N CO2Me S N Me H OH CH3 CO2Me OH H S CH2 N Me H - H N H H S H OH CH3 CO2Me CH3 CO2Me CO2Me CO2Me S N Me Toluene/ PTSA H N S 3 CH3 CO2Me 8. First Generation Cephalosporins Summary •Generally lower activity than comparable penicillins •Better range of activity than comparable penicillins •Best activity is against Gram-positive cocci •Useful against some Gram negative infections •Useful against S. aureus and streptococcal infections when penicillins have to be avoided •Poorly absorbed across the gut wall (except for 3-methyl substituted cephalosporins) •Most are administered by injection •Resistance has appeared amongst Gram negative bacteria (presence of more effective blactamases) 9. Second Generation Cephalosporins 9.1 Cephamycins H OMe H N HO2C H2N H S Cephamycin C O N O O CO2H C O •Isolated from a culture of Streptomyces clavuligerus •First b-lactam to be isolated from a bacterial source •Modifications carried out on the 7-acylamino side chain NH2 9. Second Generation Cephalosporins 9.1 Cephamycins H OMe H N 7 S O S Cefoxitin 3 N O CO2H O C NH2 O •Broader spectrum of activity than most first generation cephalosporins •Greater resistance to b-lactamase enzymes •The 7-methoxy group may act as a steric shield •The urethane group is stable to metabolism 9. Second Generation Cephalosporins 9.2 Oximinocephalosporins Me O N C O H H N O H Cefuroxime S N O O CO2H C NH2 O •Much greater stability against some b-lactamases •Resistant to esterases due to the urethane group •Wide spectrum of activity •Useful against organisms that have gained resistance to penicillin •Not active against P. aeruginosa •Used clinically against respiratory infections 10. Third Generation Cephalosporins Oximinocephalosporins R Me Aminothiazole ring O N H 2N S N C H H N O H Cef otaxime Cef tizoxime CH2OCOMe H Me S CH2S N N N R O N Cef triaxone OH O CO2H •Aminothiazole ring enhances penetration of cephalosporins across the outer membrane of Gram -ve bacteria •May also increase affinity for the transpeptidase enzyme •Good activity against Gram -ve bacteria •Variable activity against Gram +ve cocci •Variable activity vs. P. aeruginosa •Lack activity vs MRSA •Generally reserved for troublesome infections 10. Third Generation Cephalosporins Oximinocephalosporins Me Me O N C S N H 2N CO2H H H N O H Ceftazidime S N N O CO2 •Injectable cephalosporin •Excellent activity vs. P. aeruginosa and other Gram -ve bacteria •Can cross the blood brain barrier •Used to treat meningitis 11. Fourth Generation Cephalosporins Oximinocephalosporins R Me O N H 2N S Me N C H H N O H CH2 S N N R O CO2H CH2 N Cef ipime Cef pirome •Zwitterionic compounds •Enhanced ability to cross the outer membrane of Gram negative bacteria •Good affinity for the transpeptidase enzyme •Low affinity for some b-lactamases •Active vs. Gram +ve cocci and a broad array of Gram -ve bacteria •Active vs. P. aeruginosa COMPULSORY READING FROM TEXTBOOK – WILSON AND GISVOLD • PAGES - 278 TO 293 • EXAMPLES: ALL EXAMPLES LISTED [PAGES 286 – 293] COMPULSORY READING FROM TEXTBOOK – PATRICK • THE ENTIRE SECTION ON CEPHALOSPORINS IN CHAPTER 19