B. Cephalosporins, monobactams and carbapenems
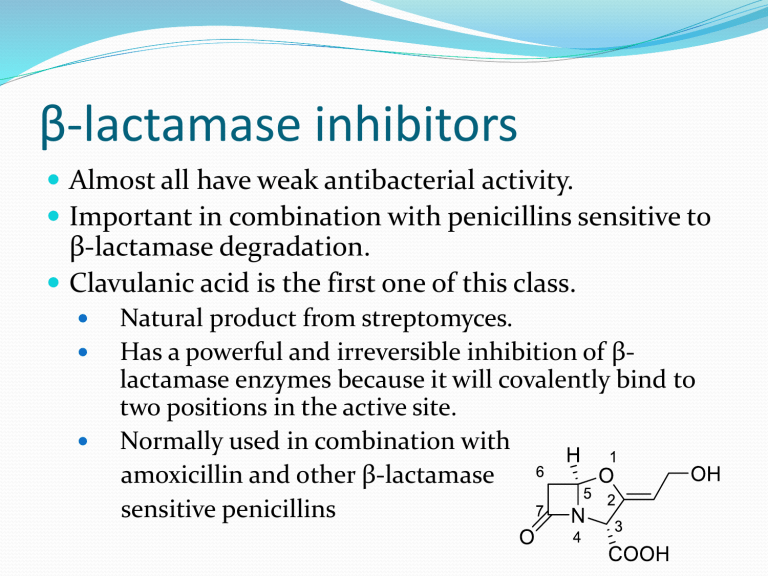
β-lactamase inhibitors
Almost all have weak antibacterial activity.
Important in combination with penicillins sensitive to
β-lactamase degradation.
Clavulanic acid is the first one of this class.
Natural product from streptomyces.
Has a powerful and irreversible inhibition of βlactamase enzymes because it will covalently bind to two positions in the active site.
Normally used in combination with amoxicillin and other β-lactamase sensitive penicillins
SAR for β-lactamase inhibitors
β-lactam ring is essential.
The enol ether have a rule in binding.
The elkene moiety should have Z configuration which is much more active than the E isomer.
No substitution at C
6
.
R stereochemistry at C
3
.
Carboxylic acid at C3 is essential.
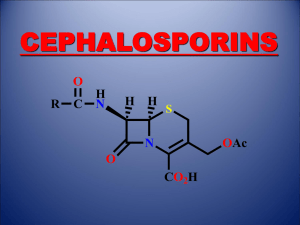
Cephalosporins.
The first agent discovered was cephalosporin C, obtained from the fungus cephalosporium acremonium.
1/1000 the antibacterial activity of penicillin G.
Has the same mechanism of action as penicillins (inhibits cell wall cross linking).
Has greater stability toward acid and β-lactamase.
SAR of cephalosporins
The bicyclic system is essential.
The carboxylic acid at C
4 is essential.
Acylamino group at C
7 is essential.
Acetyloxy group at C
3 is important and act as a leaving group when the molecule binds to transpeptidase.
Synthesis of Cephalosporins
Unlike 6-APA, 7ACA was difficult to isolate and purify.
Instead, 7ACA was synthesized from cephalosporin C as follows
1
st
Generation Cephalosporins
Have lower activity than penicillin but they have broader spectrum action.
Still susceptible to β-lactamase degradation.
Steric shield helped to improve stability toward βlactamase degradation but proved to reduce antibacterial activity.
1
st
Generation Cephalosporins
Have the good leaving group, pyridinium ion.. This improved activity.
This group is not hydrolysable compared to the acetyloxy group found in cephalothin.
Poorly absorbed from the gut because it will be ionized all the time.
Only given parenterally.
2
nd
Generation Cephalosporins
They have methoxy group at C
7 which make them active against the resistant strains.
They have a carbamate group at C
3 that increase stability toward hydrolysis compared to the acetyloxy group found in 1 st generation derivatives.
2
nd
Generation Cephalosporins
Other agents are the oximinocephalosporins:
Have the iminomethoxy group at the α-carbon in the acyl side chain, this increased stability toward βlactamase.
3
rd
Generation Cephalosporins
Here the aminothiazole ring has replaced the furan ring of cefuroxime:
This enhanced the penetration through the outer membrane of gram –ve bacteria,
Increase the affinity for transpeptidase.
Not recommended as first line therapy to prevent the rapid development of resistance.
3
rd
Generation Cephalosporins
Cefdinir (Omnicef®):
It has a broad spectrum activity.
More active on gram –ve bacterial infections such as respiratory, skin and soft tissues infections.
Estimated oral bioavailability is 20-25% (WHY?).
LogP = 0.02
pKa = 3.27
4
th
Generation Cephalosporins
They have a positively charged group at C
3 which become a good leaving group during the binding with transpeptidase.
They are more polar than the old generation, better penetration for the outer membrane of gram –ve bacteria.
More stable toward β-lactamase.
New generation Cephalosporins
Cephtobiprole
5 th generation cephalosporin (2008).
Only given IV (Why?).
Resistant to staphylococcal β-lactamase (Why?).
activity against methicillin-resistant S. aureus, penicillinresistant S. pneumoniae, P. aeruginosa, and Enterococci.
New generation Cephalosporins
Cefsulodin
3 rd generation cephalosporin.
has very specific activity against P. aeruginosa. limited activity against Gram-positive bacteria and anaerobic bacteria.
Is not clinically used nowadays (difficult to purify during synthesis).
New generation Cephalosporins
Ceftaroline
5 th generation cephalosporins.
It retains the activity of later generation cephalosporins having broad spectrum activity against Gram -ve and gram
+ve bacteria especially on resistant strains.
Approved for clinical use in USA in 2010.
Still in clinical trials (phase III).
New generation Cephalosporins
Cefmenoxime
3 rd generation cephalosporins.
It is mainly active on gram –ve bacteria.
Only available as intramuscular injections.
LogP = - 0.87
New generation Cephalosporins
Predict its pharmacokinetic and activity profile?
Carbapenems
No thiazolidine or dihydrothiazine ring.
They have two strained rings which decrease the chemical stability as well as acid stability.
The inverse stereochemistry at C enzymes.
6 and the presence of hydroxyl group increase stability toward β-lactamase
They have broad spectrum activity.
Monobactams
It has a limited activity against gram +ve bacteria.
Because it does not have the fused ring system, Aztreonam is believed to have different mechanism of action..
highly polar structure which reduce the oral bioavailability… it is recommended to be given parenterally