New C1 C2 C3 Revision
advertisement

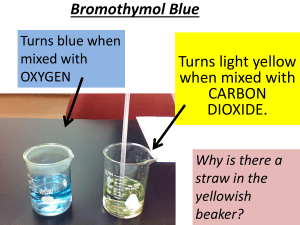
Revision C1 C2 C3 Go to C1 Go to C2 Go to C3 C1 - Air Quality Atmosphere argon carbon dioxide Respiration (adds carbon dioxide to the atmosphere) glucose + oxygen carbon dioxide + water Photosynthesis (removes carbon dioxide from the atmosphere) carbon dioxide + water glucose + oxygen Early Earth The early atmosphere consisted mainly of carbon dioxide and water vapour (probably from volcanoes), and also some nitrogen and methane. On Earth, temperatures began to cool. The water vapour gradually condensed to form the oceans. Some of the carbon dioxide began to dissolve in the oceans and later became incorporated into sedimentary rocks. Scientists looked at fossil evidence of early life. The evidence suggests that oxygen levels first increased due to early plant life. The early plants used up carbon dioxide during photosynthesis and released oxygen. The 3 main fossil fuels are: • coal • crude oil • natural gas The chemical reaction when a fuel burns is: FUEL + OXYGEN H O O H C H + O O H CARBON + WATER DIOXIDE H O H O C O + H O H Incomplete burning produces carbon monoxide During the course of a chemical reaction the numbers of atoms of each element must be the same in the products as in the reactants Atmospheric pollutants cannot just disappear, they have to go somewhere • particulate carbon is deposited on surfaces, making them dirty; • sulphur dioxide and nitrogen dioxide react with water and oxygen to produce acid rain; • carbon dioxide is used by plants in photosynthesis; • carbon dioxide dissolves in rain water and in sea water. How can atmospheric pollution caused by power stations which burn fossil fuels be reduced ? • using less electricity; • removing sulfur from natural gas and fuel oil; • removing sulfur dioxide and particulates (carbon and ash) from the flue gases emitted by coal-burning power stations How can atmospheric pollution caused by exhaust emissions from motor vehicles be reduced ? • burning less fuel by having more efficient engines; • using low sulphur fuels; • using catalytic converters, which convert nitrogen monoxide to nitrogen and oxygen, and carbon monoxide to carbon dioxide; • adjusting the balance between public and private transport; • having legal limits to emissions (which are enforced by the use of MOT tests). Burning of fuel > sulphur dioxide gas > reacts with water vapour > acid rain (which contains sulfur eg coal) hydrogen carbon monoxide oxygen sulfur dioxide sulfur carbon dioxide nitrogen nitrogen monoxide carbon nitrogen dioxide water Sample Test 1 Test 2 Test 3 A 35 36 37 B 36 33 35 C 32 26 34 D 35 36 38 Range = lowest value to the highest value 108 / 3 36 outlier Eg for sample C including 32 to 34 Not the outlier The average / mean is calculated to smooth out small differences in the data. It is the best estimate of the true value. When asked to evaluate data, make reference to its reliability (i.e. is it repeatable ? Range 2 These ranges do not overlap so there is a real difference. Range 1 Range 2 Range 1 These ranges do overlap so there is not a real difference. How does air quality affect our health? To find out what causes hayfever it is important to first look at what factors (pollen) are linked with hayfever. Factors are variables that may affect the outcome (hayfever). If an outcome increases (or decreases) as a particular factor increases, this is called a correlation. Evidence shows there is a correlation between pollen levels and hayfever symptoms, but is pollen the cause of hayfever? Another correlation is if the concentration of nitrogen dioxide stays high for several days, there is an increased risk of people with asthma suffering from asthma attacks – but does nitrogen oxide cause asthma Continue with Foundation Go to Higher Spend 10 min writing down everything you can remember about C1 oxygen argon nitrogen C (61+63+65+62+64) 5 63 hydrocarbons carbon dioxide or water sulphur dioxide Go to C1 Go to C2 Go to C3 Higher material can justify the claim that there is/is not a ‘real difference’ between two measurements of the same quantity; recall that nitrogen monoxide NO, is formed during the combustion of fuels in air, and is subsequently oxidised to nitrogen dioxide NO2 .(NO and NO2 are jointly referred to as ‘NOx’); can explain why scientists regard it as important that a scientific claim can be replicated by other scientists; can explain why a correlation between a factor and an outcome does not necessarily mean that one causes the other, and can give an example to illustrate this; Spend 10 min writing down everything you can remember about C1 hydrocarbons carbon dioxide or water E D A B Acid rain Go to C1 Go to C2 Go to C3 C2 - Material Choices Quick Overview Properties of materials – tensile strength, shear strength, hardness, flexibility, elasticity, melting point, absorbency Testing materials – fair test, reliability, accuracy, range, mean, outlier, real difference Changing properties of polymers – chain length, density, cross linking, plasticizers Natural and synthetic Life cycle assessment – energy used, materials used, resources used, effects on the environment Crude Oil Properties of materials – tensile strength, shear strength, hardness, flexibility, elasticity, melting point, absorbency tensile strength – when trying to pull it shear strength – when trying to tear it absorbency – ability to absorb liquid melting point – temperature at which a solid becomes a liquid Testing materials – fair test, reliability, accuracy, range, mean, outlier, real difference How do you make data more reliable ? • do repeat measurements • don’t include outliers • use mean values How do you make data more accurate ? • use more precise instruments Range 2 Range 2 Range 1 Range 1 These ranges do not overlap so there is a real difference. These ranges do overlap so there is not a real difference. Key Words throwing something away disposal landfill burying rubbish burning rubbish incineration can be broken down naturally in the environment biodegradable renewable a source which will not run out non-renewable reusable recyclable a source which will run out can be used again (after cleaning) as something useful can be broken down/melted and be made into something useful Why is it better to use data rather than opinion in justifying a position ? > because it is more convincing to use verifiable evidence How may a measurement be inaccurate ? > eg faulty equipment, human error etc How can several measurements of the same quantity give different results ? > the accuracy of the equipment ; the quantity has a natural variation What key thing makes data reliable ? > when it is repeatable The mean is the best estimate of the true value. What is an outlier ? > data which is much higher or lower than all of the other values Why are outliers often discarded ? > because they are usually errors What is a fair test ? > when you only change one factor (independent variable) and control the others by keeping them the same and then measure the outcome (dependent variable). The hydrocarbon molecules in crude oil vary in size. methane butane The larger the molecule: • the greater the number of carbon atoms • the higher its boiling point • the less volatile it is • the less easily it flows ( the more viscous it is ) • the less easily it ignites ( the less flammable it is ) Polymerisation is the joining together of monomers to make polymers Polymerisation using 2 different types of monomer produces a polymer with different properties polymer monomers Conditions - Heat & a Catalyst ethene polyethene (polythene) Only a small percentage of crude oil is used for chemical synthesis, what is the rest of it used for ? > fuels and lubricants The petrochemical industry refines crude oil to produce fuels, lubricants and the raw materials for chemical synthesis fuel chemical synthesis fuel fuel fuel fuel & lubricants fuel & lubricants What affects the temperature at which a substance melts ? > the greater the strength of the forces between the particles the higher the amount of energy needed to break them out of the solid structure branching cross linking high density B A D C E plasticiser chain length branching cross linking high density / crystallinity plasticisers chain length depends on the branching increases strength, more rigid, stops molecules sliding past each other increases strength, hardness and density, molecules closer together more flexible, easier to bend, allows molecules to slide past each other the longer the stronger Natural fabrics wool plant cotton animal silk plant leather insect rubber sheep Why are mixtures of fabrics often used, eg cotton and polyester for a shirt ? > to take advantage of useful properties, eg cotton is good at absorbing sweat and polyester will make it more stretchable Wood versus Plastic ! > wood uses a renewable resource while plastic involves a non-renewable resource (ie crude oil), however, wood rots where as plastic doesn’t rot Nanotechnology – the use and control of structures called nanoparticles on a tiny (nanometre) scale. Nanometre –a unit of length 1000000000 times smaller than a metre Nanoparticles of a material show different properties to larger particles of the same material. Uses of nanotechnology •Sunscreen •Tennis balls and rackets •Electronic paper •Clothing •Self-cleaning windows Continue with Foundation Go to Higher x x refined hydrocarbons x x x x x softer stronger stronger x x Go to C1 Go to C2 Go to C3 can justify the claim that there is/is not a ‘real difference’ between two measurements of the same quantity; can explain why it is necessary to control all factors thought likely to affect the outcome other than the one being investigated, ie can explain a fair test can explain why different courses of action may be taken in different social and economic contexts. 4 x x x x x softer x x x x x x x x Go to C1 Go to C2 Go to C3 C3 - Chemicals in our lives: risks and benefits A journey through geological time The Earth’s outer layers are divided into a number of tectonic plates. The plates move because of very slow convection currents in the underlying solid mantle. Magnetic clues in rocks help geologists to track the very slow movement of the continents Processes such as mountain building, erosion, sedimentation, dissolving, and evaporation have led to the formation of valuable minerals. Clues to the conditions under which rocks were formed come from fossils, shapes of sand grains, and ripples made by flowing water. Salt (sodium chloride) – sources and uses Uses Sea salt – large-scale •Preserving food extraction of salt from •Processing food the sea is only economical •Source of chemicals e.g. chlorine on coasts with hot and dry •Treat roads in winter climates. Rock salt – contains about 90% sodium chloride in the mineral halite. Solution mining –salt used for the chemical industry is extracted by pumping water down into the rock called brine. Salt crystals are recovered by evaporating the water. Salt in food –used as flavouring. Eating too much salt can raise people’s blood pressure, this can increase the risk of developing heart disease or having a stroke Alkalis and their uses Alkalis were needed in pre-industrial times •To neutralise acid soils •Convert fats and oils into soap •Make glass Traditional sources of alkalis were •Burnt wood •Stale urine •Make chemicals that bind natural dyes to cloth Alkalis are important because they neutralise acids. Sodium hydroxide + hydrochloric acid sodium chloride + water (alkali) (acid) (salt) Hydrochloric acid produces chloride salts Sulfuric acid produces sulfate salts Nitric acid produces nitrate salts Alkaline carbonate + acid salt + carbon dioxide + water Making alkali on a large scale • Leblanc invented a process in 1791 that used chalk, salt and coal to make the alkali sodium carbonate. •The Leblanc process was highly polluting. •Parliament passed the Alkali Acts in 1863 to tackle the pollution problem. Benefits and risks of water treatment Chlorination of drinking water in Britain became increasingly common in the early 20th century, this led to a steep decline in deaths from typhoid. Some scientists are concerned that chlorination can lead to the formation of trihalomethanes (THMs). There is a suspicion that THMs could lead to some forms of cancer, but research studies have not found any firm evidence to support this idea.. The electrolysis of brine Manufacturing chemicals from salt by electrolysis needs a lot of energy Uses of chemicals from salt Sodium hydroxide Chlorine •Make bleach •Treat drinking water •Make soap and paper •Make bleach •Process food products •Make HCl •Remove pollutants from water •Make plastics •Chemical processing and products •Make fibres •Make solvents Hydrogen •Make hydrochloric acid •Fuel to produce steam Protecting health and the environment REACH – introduced in 2007 by the EU to collect information about the hazards of chemicals and to assess the risks. POPs – persistent organic pollutants. These are organic compounds that do not break down in the environment for a very long time. They can spread widely around the world and build up in fatty tissue of humans and animals. They can be harmful to people and the environment. Stages in the life of PVC (synthetic polymer) Chlorine and ethene are combined to make vinyl chloride (can cause cancer) Vinyl chloride molecules are joined to make long chains of PVC poly(vinyl chloride) by polymerisation PVC granules are sent to factories to be moulded under heat and pressure. About half of PVC is used to make pipes (carry drinking water, sewage and gas). A softer type is used to make clothing and garden hoses. The best way to get rid of old PVC is recycling. Some polymer waste can be burnt in special incinerators. Unfortunately most ends up in landfill The properties of PVC can be altered by plasticisers Plasticisers added to PVC can leach out from the plastic into the surroundings where they may have harmful effects. Go to C1 Go to C2 Go to C3