Stratospheric chemistry - Atmospheric Chemistry Modeling Group
advertisement

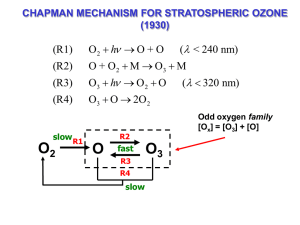
CALCULATION OF PHOTOLYSIS RATES X h ... d[ X ] k[ X ] dt k is the photolysis rate constant (also called photolysis frequency or J-value) k q X ( ) X ( ) I ( ) d 0 quantum yield absorption x-section actinic flux (omnidirectional) photon is not absorbed Probability of absorption for incoming photons = σ/A Absorption crosssection photon is absorbed Molecular cross-section A CALCULATION OF 3-BODY REACTION RATES A B AB * (1) AB* A B (2) AB * M AB M * (3) M * M heat (4) Net: A B M AB M General solution: A and B are reactants; AB* is the activated product; AB is the stable product; M is the “third body” (N2, O2 ) d [ AB] k1k3[ A][ B][ M ] dt k2 k3[ M ] Low-pressure limit (Rate(2) >> Rate (3)): High-pressure limit (Rate(2) << Rate (3)): d [ AB] k1k3 [ A][ B][ M ] dt k2 d [ AB] k1[ A][ B] dt THE OZONE LAYER 1 Dobson Unit (DU) is defined to be 0.01 mm thickness at STP Latest satellite ozone data: http://jwocky.gsfc.nasa.gov/ ABSORPTION OF SOLAR UV RADIATION BY OZONE Solar UV radiation spectrum at different altitudes THE NATURAL OZONE LAYER Based on ozonesonde observations in the 1970s SOLAR SPECTRUM AND ABSORPTION X-SECTIONS O2+hv O3+hv ENERGY STATES OF THE O ATOM (1s22s22p4) determined by the arrangement of the four electrons in the 2p orbitals multiplicity total electronic orbital angular momentum number Multiplicity = 2S+1, where S is the spin. The spin of an electron is (+/‐) 1/2. .. Hund’s Rule: lowest-lying energy state is the one of maximum multiplicity 94 kJ/,ole : O: .. Energy O(1 S) O(1D) O(3P) CHAPMAN MECHANISM FOR STRATOSPHERIC OZONE (1930) (R1) O 2 h O + O (R2) O + O 2 M O3 M (R3) O3 h O 2 O (R4) O3 O 2O 2 slow O2 R1 fast R3 R4 slow ( 320 nm) Odd oxygen family [Ox] = [O3] + [O] R2 O ( < 240 nm) O3 STEADY-STATE ANALYSIS OF CHAPMAN MECHANISM Lifetime of O atoms: [O] 1 O k2 [O][O2 ][M]+k4 [O3 ][O] k2CO2 na2 1s …is sufficiently short to assume steady state for O: k3 O [O] R 2 R3 k2 [O][O2 ][M]=k3[O3 ] 2 [O3 ] k2CO 2 na O3 [Ox ] [O3 ] …so the budget of O3 is controlled by the budget of Ox. Lifetime of Ox: Ox [Ox ] 1 2k4 [O3 ][O] 2k4 [O] Steady state for Ox: τOx 1 2 3 k1k2 2 2R1 2R4 k1[O2 ] k4 [O3 ][O] [O3 ] CO2 na k3k4 1 PHOTOLYSIS RATE CONSTANTS: VERTICAL DEPENDENCE X+h ... k qX ( ) X ( ) I d 0 I ( z dz ) optical depth d ( O2nO2 ( z) O3nO3 ( z))dz I ( z) I ( z) I () e ( O 2 nO 2 ( z ') O3nO3 ( z '))dz ' z quantum yield absorption X-section photon flux CHAPMAN MECHANISM vs. OBSERVATION shape determined by k1nO2 -3 Chapman mechanism reproduces shape, but is too high by factor 2-3 e missing sink! EVOLUTION OF O2 AND O3 IN EARTH’S ATMOSPHERE Questions 1. Show that the loss of ozone in the Chapman mechanism depends quadratically on the ozone concentration, i.e., L(O3) ~ [O3]2 2. The production of ozone by photolysis of O2, P(O3) = k1[O2], appears to depend linearly on the O2 concentration but the dependence is in fact much weaker than linear. Explain why. RADICAL REACTION CHAINS IN THE ATMOSPHERE Initiation: non-radical Propagation: radical + non-radical Termination: radical + radical radical + radical + M radical + radical photolysis thermolysis oxidation by O(1D) non-radical + radical bimolecular redox reactions non-radical + non-radical radical redox reaction non-radical + M 3-body recombination WATER VAPOR IN STRATOSPHERE H2O mixing ratio Source: transport from troposphere, oxidation of methane (CH4) Ozone loss catalyzed by hydrogen oxide (HOx ≡ H + OH + HO2) radicals H2O + O( D) 2OH 1 Initiation: OH + O3 HO2 O2 Propagation: HO2 + O3 OH + 2O2 Net: Termination: 2O3 3O2 OH + HO2 H2O + O2 slow H2O OH fast HO2 slow HOx radical family Supersonic aircraft (Concorde) cruising at 60,000’ Questions 1, A sink for HOx radicals in the stratosphere is formation of hydrogen peroxide (H2O2): HO2 HO2 H 2O2 O2 H2O can then go on to either photolyze or react with OH: H 2O2 h 2OH H 2O2 OH H 2O HO2 Is this an effective termination pathway for HOx-catalyzed ozone loss? 2. Write a catalytic cycle of propagation reactions starting with the reaction HO2 NO OH NO2 and based on the reactions we have seen so far. Does your cycle destroy ozone or is it a null cycle? WHAT IS A RATE-LIMITING STEP? • From IUPAC: “A rate-controlling (rate-determining or rate-limiting) step in a reaction occurring by a composite reaction sequence is an elementary reaction the rate constant for which exerts a strong effect — stronger than that of any other rate constant — on the overall rate.” It is not necessarily the slowest reaction in the sequence! NITROUS OXIDE IN THE STRATOSPHERE H2O mixing ratio ATMOSPHERIC CYCLING OF NOx AND NOy STRATOSPHERIC OZONE BUDGET FOR MIDLATITUDES CONSTRAINED FROM 1980s SPACE SHUTTLE OBSERVATIONS STRATOSPHERIC DISTRIBUTION OF CF2Cl2 (CFC-12) ATMOSPHERIC CYCLING OF ClOx AND Cly SOURCE GAS CONTRIBUTIONS TO STRATOSPHERIC CHLORINE (2004) CHLORINE PARTITIONING IN STRATOSPHERE Decrease of Cl-containing gases following Montreal protocol = 45 years = 100 years = 5 years = 26 years • Original Montreal protocol (1987): cap production rates at 1980s levels • London (1990), Copenhagen (1992) amendments: phase-out in developed world • Beijing (1999): worldwide ban on production Questions 1. It has been argued that a fleet of supersonic aircraft releasing NOx in the lower stratosphere would decrease chlorine-catalyzed ozone loss. Why? [Hint: think of the chlorine reservoirs] 2. Peroxynitric acid (HNO4) is produced and removed in the stratosphere by HO2 + NO2 + M → HNO4 + M HNO4 + OH → H2O + NO2 + O2 What is the effect on stratospheric ozone? Think of the effects on both the NOx and HOx budgets. 3. Photochemical model calculations for the stratosphere including only the Chapman mechanism overestimate observed ozone levels by a factor of 3. However, in a budget calculation constrained by ozone observations we find that the O3 + O reaction accounts for only 10% of the Ox sink. Can you reconcile these two results? OZONE TREND AT HALLEY BAY, ANTARCTICA (OCTOBER) Farman et al. paper published in Nature 1 Dobson Unit (DU) = 0.01 mm O3 STP = 2.69x1016 molecules cm-2 SPATIAL EXTENT OF THE OZONE HOLE Mean October data Movie of October 1979-2013 Antarctic ozone http://ozonewatch.gsfc.nasa.gov/ Isolated concentric region around Antarctic continent is called the polar vortex. Strong westerly winds, little meridional transport THE OZONE HOLE IS A SPRINGTIME PHENOMENON Movie of the 2013 ozone hole VERTICAL STRUCTURE OF THE OZONE HOLE: near-total depletion in lower stratosphere Argentine Antarctic station southern tip of S. America High ClO in polar vortex Sept. 1987 ER-2 aircraft measurements at 20 km altitude south of Punta Arenas O3 ClO O3 Sep. 16 Sep. 2, 1987 ClO 20 km altitude Measurements by Jim Anderson’s group (Harvard) Edge of Polar vortex SATELLITE OBSERVATIONS OF ClO IN THE SOUTHERN HEMISPHERE STRATOSPHERE WHY THE HIGH ClO IN ANTARCTIC VORTEX? Release of chlorine radicals from reactions of reservoir species in polar stratospheric clouds (PSCs) PSC FORMATION AT COLD TEMPERATURES PSC formation Frost point of water Seasonal PSCs in the Antarctic stratosphere HOW DO PSCs START FORMING AT 195K? HNO3-H2O PHASE DIAGRAM Antarctic vortex conditions PSCs are not water but nitric acid trihydrate (NAT) clouds DENITRIFICATION IN THE POLAR VORTEX: SEDIMENTATION OF PSCs CHRONOLOGY OF ANTARCTIC OZONE HOLE Chronology of 2013 ozone hole http://ozonewatch.gsfc.nasa.gov/ Increasing CO2 cools the stratosphere 15 m (220 K) Add CO2 to stratosphere (T2 ). At 15 m: f T 1 4 f T 2 4 f T 2 4 T1 = 220 K Net heating = f(T14 - 2T24 ) < 0 Greenhouse gases warm the surface but cool the stratosphere Questions 1. What ratio of HCl to ClNO3 concentrations in Antarctic fall will lead to the largest ozone depletion the following spring? 2. Satellite observations of ClO in the Antarctic stratosphere in the middle of winter show a "collar" of maximum values around 60 degrees S. Why isn't ClO highest over the South Pole, where temperatures are lowest? Correlation of Arctic ozone loss with temperature Unusually cold Arctic stratosphere in spring 2011 2011 Arctic ozone hole SKIN CANCER EPIDEMIOLOGY PREDICTIONS