Lecture Set 07
advertisement

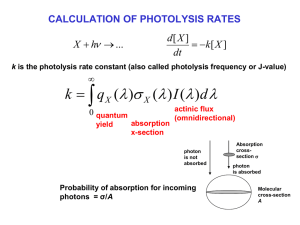
CHAPMAN MECHANISM FOR STRATOSPHERIC OZONE (1930) (R1) O 2 h O + O (R2) O + O 2 M O3 M (R3) O3 h O 2 O (R4) O3 O 2O 2 slow O2 R1 fast R3 R4 slow ( 320 nm) Odd oxygen family [Ox] = [O3] + [O] R2 O ( < 240 nm) O3 STEADY-STATE ANALYSIS OF CHAPMAN MECHANISM Lifetime of O atoms: [O] 1 O k2 [O][O2 ][M]+k4 [O3 ][O] k2CO2 na2 1s …is sufficiently short to assume steady state for O: k3 O [O] R 2 R3 k2 [O][O2 ][M]=k3[O3 ] 2 [O3 ] k2CO 2 na O3 [Ox ] [O3 ] …so the budget of O3 is controlled by the budget of Ox. Lifetime of Ox: Ox [Ox ] 1 2k4 [O3 ][O] 2k4 [O] Steady state for Ox: Ox 1 2 3 k1k2 2 2R1 2R4 k1[O2 ] k4 [O3 ][O] [O3 ] CO2 na k3k4 1 SOLAR SPECTRUM AND ABSORPTION X-SECTIONS O2+hv O3+hv PHOTOLYSIS RATE CONSTANTS: VERTICAL DEPENDENCE X+h ... k qX ( ) X ( ) I d 0 I ( z dz ) optical depth d ( O2nO2 ( z) O3nO3 ( z))dz I ( z) I ( z) I () e ( O 2 nO 2 ( z ') O3nO3 ( z '))dz ' z quantum yield absorption X-section photon flux CHAPMAN MECHANISM vs. OBSERVATION shape determined by k1nO2 -3 Chapman mechanism reproduces shape, but is too high by factor 2-3 e missing sink! RADICAL REACTION CHAINS IN THE ATMOSPHERE Initiation: non-radical Propagation: radical + non-radical Termination: radical + radical radical + radical + M radical + radical photolysis thermolysis oxidation by O(1D) non-radical + radical bimolecular redox reactions non-radical + non-radical radical redox reaction non-radical + M 3-body recombination WATER VAPOR IN STRATOSPHERE H2O mixing ratio Source: transport from troposphere, oxidation of methane (CH4) Ozone loss catalyzed by hydrogen oxide (HOx ≡ H + OH + HO2) radicals H2O + O( D) 2OH 1 Initiation: OH + O3 HO 2 O 2 Propagation: HO2 + O3 OH + 2O2 Net: Termination: 2O3 3O2 OH + HO2 H2O + O2 slow H2O OH fast HO2 slow HOx radical family NITROUS OXIDE IN THE STRATOSPHERE H2O mixing ratio Ozone loss catalyzed by nitrogen oxide (NOx ≡ NO + NO2) radicals • Initiation N2O + O(1D) 2NO • Propagation NO + O3 NO2 + O2 NO + O3 NO2 + O2 NO2 + h NO + O NO2 + O NO + O2 O + O2 + M O3 + M O3 loss rate: Null cycle Net O3 + O 2O2 d [O3 ] 2k[NO ][O] 2 dt • Termination Recycling NO2 + OH + M HNO3 + M HNO3 + h NO2 + OH NO2 + O3 NO3 + O2 HNO3 + OH NO3 + H2O NO3 + NO2 + M N2O5 + M NO3 + h NO2 + O N2O5 + H2O 2HNO3 N2O5 + h NO2 + NO3 ATMOSPHERIC CYCLING OF NOx AND NOy STRATOSPHERIC OZONE BUDGET FOR MIDLATITUDES CONSTRAINED FROM 1980s SPACE SHUTTLE OBSERVATIONS Gas-phase chemistry only STRATOSPHERIC DISTRIBUTION OF CF2Cl2 (CFC-12) Ozone loss catalyzed by chlorine (ClOx ≡ Cl + ClO) radicals • Initiation: Cl radical generation from non-radical precursors (e.g., CFC-12) CF2Cl2 + h g CF2Cl + Cl • Propagation: Cl + O3 g ClO + O2 ClO + O g Cl + O2 Net: O3 + O g 2O2 • Termination: Cl + CH4 g HCl + CH3 ClO + NO2 + M g ClNO3 + M d [O3 ] 2k[ClO][O] O3 loss rate: dt Recycling: HCl + OH gCl + H2O ClNO3 + h gCl + NO3 ATMOSPHERIC CYCLING OF ClOx AND Cly SOURCE GAS CONTRIBUTIONS TO STRATOSPHERIC CHLORINE (2004) CHLORINE PARTITIONING IN STRATOSPHERE OZONE TREND AT HALLEY BAY, ANTARCTICA (OCTOBER) Farman et al. paper published in Nature 1 Dobson Unit (DU) = 0.01 mm O3 STP = 2.69x1016 molecules cm-2 SPATIAL EXTENT OF THE OZONE HOLE Isolated concentric region around Antarctic continent is called the polar vortex. Strong westerly winds, little meridional transport THE OZONE HOLE IS A SPRINGTIME PHENOMENON VERTICAL STRUCTURE OF THE OZONE HOLE: near-total depletion in lower stratosphere Argentine Antarctic station southern tip of S. America ASSOCIATION OF ANTARCTIC OZONE HOLE WITH HIGH LEVELS OF CLO Sept. 1987 ER-2 aircraft measurements at 20 km altitude south of Punta Arenas O3 ClO O3 Sep. 16 Sep. 2, 1987 ClO 20 km altitude Measurements by Jim Anderson’s group (Harvard) Edge of Polar vortex SATELLITE OBSERVATIONS OF ClO IN THE SOUTHERN HEMISPHERE STRATOSPHERE WHY THE HIGH ClO IN ANTARCTIC VORTEX? Release of chlorine radicals from reactions of reservoir species in polar stratospheric clouds (PSCs) PSC FORMATION AT COLD TEMPERATURES PSC formation Frost point of water HOW DO PSCs START FORMING AT 195K? HNO3-H2O PHASE DIAGRAM Antarctic vortex conditions PSCs are not water but nitric acid trihydrate (NAT) clouds DENITRIFICATION IN THE POLAR VORTEX: SEDIMENTATION OF PSCs CHRONOLOGY OF ANTARCTIC OZONE HOLE TRENDS IN GLOBAL OZONE Mt. Pinatubo LONG-TERM COOLING OF THE STRATOSPHERE Sep 21-30, 25 km, 65-75˚S Increasing CO2 is expected to cool the stratosphere TRENDS IN POLAR OZONE Could greenhouse-induced cooling of stratosphere produce an Arctic ozone hole over the next decade? Race between chlorine decrease and climate change SKIN CANCER EPIDEMIOLOGY PREDICTIONS