Ozone Depletion
advertisement

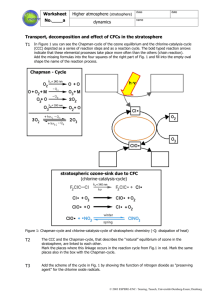
Ms. Pride Regents Biology Human Impact Presentation Ozone (O3) : The ozone layer is a layer of the upper atmosphere that blocks out harmful UV rays. Between 20 and 50 kilometers above the Earth’s surface Absorbs most of the harmful UV light from sunlight before it reaches the Earth’s surface Ozone depletion: the gradual thinning of the ozone layer and the decline of the total amount of ozone remaining in the Earth's atmosphere The main cause of ozone layer depletion was the emission of CFCs (Chloroflurocarbons) containing chlorine. CFCs escape into the atmosphere from refrigeration and propellant devices and other chemical processes CFCs eventually end up in the stratosphere, where UV light breaks the bond holding chlorine atoms (Cl) to the CFC molecule. A free chlorine atom goes on to participate in a series of chemical reactions that both destroy ozone and return the free chlorine atom to the atmosphere to cause more damage. These are all the major forms of chlorine being put into the stratosphere. Predictions of chlorine concentrations in the stratosphere. These predictions lead to policy reform. Extent of the Problem: Average yearly size of the ozone hole This shows how the size of the ozone hole varies year to year, and that recently the trend has been that the hole has fluctuated in size. Health Effects as a Result of Ozone Layer Depletion UV light is a major factor to skin cancer: UV light damages DNA Hole is centered over Antarctica and over the Artic If holes start to form over areas like cities and beaches, there will be a rapid increase in the number of patients with skin cancer. UV light also affects plant vascular tissue. Plankton is one of the most important parts of a fundamental aquatic food chain. If plankton were to become extinct because eof an inability to reproduce and pass in genetic information, then almost all the other organisms in the aquatic ecosystem would be effected. Solutions and Policy Reform Montreal Protocol (meeting of forty-six nations) called for the immediate resduction in production and use of CFCs by the year 2000. US passed law to ban use of CFCs in aerosol cans by 2000. Check to ensure aerosols and refrigeration chemicals are CFC-free. http://ozonewatch.gsfc.nasa.gov/index.html http://www.cpc.ncep.noaa.gov/products/stratosphere/winter_b ulletins/sh_07/Fig_3.gif http://en.wikipedia.org/wiki/Image:Sources_of_stratospheric_c hlorine.png http://www.epa.gov/ozone/ http://www.atm.ch.cam.ac.uk/tour/part3.html http://www.nas.nasa.gov/About/Education/Ozone/history.htm l http://www.cpc.ncep.noaa.gov/products/stratosphere/winter_b ulletins/sh_07/Fig_3.gif