(R 2 Si) n
advertisement
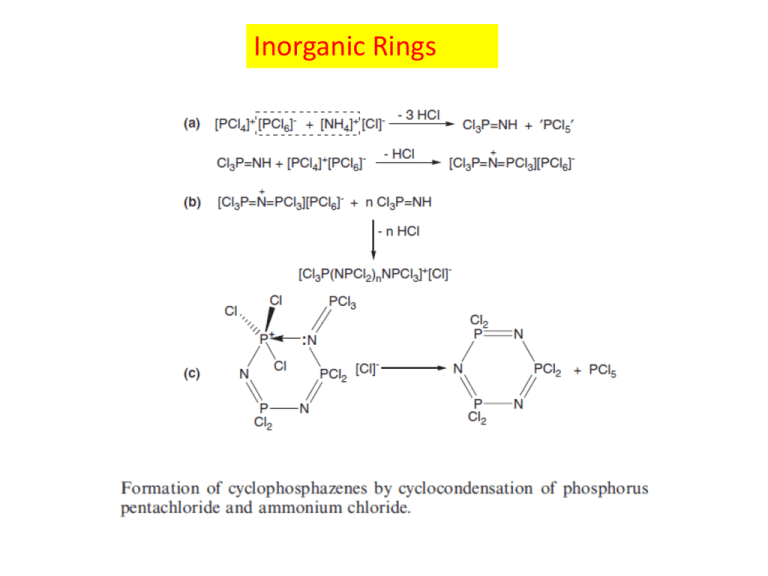
Inorganic Rings Borazine: “inorganic” benzene planar six-membered ring with endocyclic bond angles of ca 118o at boron and ca 121o at nitrogen and equal B–N bond lengths of 1.43A ˚ , which lie between those of typical single and double boron–nitrogen bonds. In contrast to benzene, for which each carbon atom contributes one electron to the π-system, each nitrogen in borazine has two electrons in the pπ-orbital while the boron pπ-orbitals are vacant. Cyclophosphazenes Form an extensive homologous series (NPX2)n (n=3–40; X=F). The six-membered rings are usually planar (or close to planar), whereas the larger ring systems adopt puckered conformations, with the exception of the tetramer (NPF2)4, which is almost planar. The parent system (X=H) is not known, but a wide variety of derivatives where X=Cl, F, CF3, alkyl, OR, NR2 The P atoms in cyclophosphazenes use four valence electrons in forming σ-bonds to their four nearest neighbours, leaving one electron available for π-bonding. The N atoms utilize two electrons to bond to the two adjacent phosphorus atoms and also accommodate a lone pair of electrons in an ‘sp2’ orbital in the plane of the P3N3 ring. Thus, each N atom has one electron available for π-bonding in a p-orbital perpendicular to the plane of the ring. Τhe cyclotriphosphazenes, (NPX2)3 are π-electron precise systems with six π-electrons for six ring atoms. Sulfur-Nitrogen Rings In 1972, it was proposed that these S–N heterocycles belong to a class of ‘electron-rich aromatics’ which conform to the Hückel 4n+2 π-electron rule Εach S and each N atom uses two valence e- for bonding in the σ-system and that there is a lone pair on each atom in the plane of the ring, this proposal was based on the contribution of two electrons from each S and one electron from each N to the π-system. Paramagnetic Inorganic Rings Cyclopolysilanes (R2Si)n (n=4–35) the first example, the hexamer (Me2Si)6, was reported in 1949 by the General Electric Co. Laboratories The standard synthesis of permethylated cyclopolysilanes (Me2Si)n (n=5–35) involves the reductive coupling of dimethyldichlorosilane, Me2SiCl2, with sodium-potassium alloy in THF, which, under thermodynamically controlled conditions, gives primarily the hexamer (Me2Si)6 (90%). Cyclosiloxanes (R2SiO)n Early studies on the phenyl derivative Ph2SiCl2 were carried out in an attempt to generate the monomer Ph2Si=O, the silicon analogue of a ketone; hence they were referred to as ‘silico(keto)nes’. This work led to the characterisation of both six- and eight-membered rings (Ph2SiO)n (n=3, 4), which are high-melting white solids. There is an interesting analogy between cyclosiloxanes and the cyclic metasilicates [SinO3n]2n- (n=3–6), e.g. (Me2SiO)3 (10.42) and [Si3O9]6- (10.43); the methyl groups in the dimethylsiloxanes are replaced by the formally isoelectronic O- in the silicates Silicon-Oxygen Polymers: Polysiloxanes (Silicones) Polysiloxanes were first developed in the 1930s and 1940s and currently represent the most commercially important inorganic polymer system. These materials and their products comprise a billion dollar global industry The exceptional properties of polysiloxanes are a direct result of their inorganic backbone of silicon and oxygen atoms and have resulted in their widespread use as highperformance elastomers and fluids, surface modifiers, adhesives and biomedical materials. Si–O bonds are stronger than C–C bonds (bond energies: Si–O ca 450 kJ mol1, C–C ca 348 kJ mol-1) and are more stable to oxidation and UV radiation Unique dynamic flexibility. This leads to materials which retain elasticity and do not become brittle even at very low temperatures. Polysiloxanes also possess a variety of other useful properties such as hydrophobicity and exceptionally high permeability to gases soft contact lenses and artificial skin Industrial Applications Industrial interest in inorganic ring systems was stimulated in the 1940s by the discovery that cyclosiloxanes, (R2SiO)n (n=3, 4), are important intermediates in the manufacture of silicone (siloxane) polymers, (R2SiO)n. Today, these inorganic polymers are made on a massive scale annually because of their multifarious uses in modern society as oils, greases, rubbers, polishes, coatings and insulating materials. Polysilanes, (R2Si)n, are of interest as ceramic precursors and in the application of microlithography in the electronics industry. Phosphazene polymers, (NPR2)n (R=HNMe, OCH2CF3, OC6H5), have many desirable properties that have led to a variety of significant applications, e.g. as water repellents, non-flammable fibres, foams, fuel pipes and metal ion conductors in batteries. In addition to their importance as precursors to inorganic polymers, more recent applications of inorganic ring systems have focused on their use as sources of functional inorganic materials such as semiconductors and ceramics.