PowerPoint Presentation - Nuclear Magnetic Resonance
advertisement

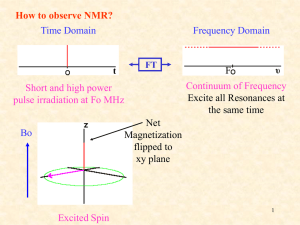
Nuclear Magnetic Resonance -an analytical tool in Physical ChemistryTodays lecture •Physical underpinnings to NMR •Integration and quantitative aspects •The Chemical Shift •The interplay of kinetic and equilibria phenomena in NMR measurements. •The investigation of an equilibrium behavior in the Physical Chemistry Lab. Before class/lab, read and understand the lab writeup for the NMR experiment. Background Reading Go to the link below: http://astro.temple.edu/%7Edebrosse/ Here, read specifically: •the UserGuide for the Inova300 •Guide to moving NMR data to PCs •UserGuide for NUTS, the offline NMR data processing software Also read: •The PChem lab writeup “NMRPchemLab.doc” What is NMR Spectroscopy? • Nuclear Magnetic Resonance • Radio Frequency Absorption Spectra of atomic nuclei in substances subjected to magnetic fields. • Spectral Dispersion is Sensitive to the chemical environment via “coupling” to the electrons surrounding the nuclei. • Interactions can be interpreted in terms of structure, bonding, reactivity NMR-What is it Good For? (absolutely everything!) • Solving structures of compounds like synthetics, impurities, natural Based on what products you know from • Identifying metabolites sophomore • Stereochemical determination • Follow reactions organic, you • Validating electronic theory; trends within series of compds. would think that • Kinetics NMR is really • Extended structure, e.g. protein nmr just for • Molecular interactions e.g. ligand binding fingerprinting • Acid-base questions organic • Purities structures, and • Mechanisms, e.g. isotope distributions, other effects determining • Questions about the solid state their structures! • Imaging • Todays’s focus, NMR as an Analytical Tool for quantifying mixtures What are the Measurables in NMR? • Intensity (analytical parameter, proportional to molarity) • Chemical Shift (the electronic surroundings) • Couplings (scalar J and dipolar D; bond paths, angles connectivity and distances) • Relaxation parameters (motions, distances) Origin of the NMR Effect • Nuclei with other than A(#protons+neutrons) and Z(#protons) both even numbers, possess net spin and associated angular momenta • Reveals itself only in magnetic field. As usual, such momenta are quantized • States have different energies, populated according to Boltzmann distribution • States are 1/2, 3/2, 5/2…for A= odd number and integer if A= even number and Z= odd number • Transitions of individual nuclei between spin states is possible (both directions) leading to an equilibrium of populations • Number of states is 2I + 1 • Many elements have NMR active nuclei. • Those elements like 1H, 13C, 31P, 19F are the most popular and accessible because they have spin I = 1/2, and this makes their NMR signals narrow and relatively easy to measure and interpret. Why NMR? • • • • • • Unmatched versatility as an Analytical technique High on chemical information content Significant interpretability Interpretable at several levels of sophistication Response related to molar preponderance These attributes are true for solids, liquids, mixtures, and to a small extent, gas phase • More than half the periodic table has at least one NMR active isotope But on the other Hand… • NMR is one of the least sensitive analytical methods • Characterized by long relaxation time constants, limiting experimental efficiency in real time • Sometimes too much information. Can be demanding on interpretation skill • Relatively Expensive compared with other analytical methods • As with other methods NMR has “blind spots” and cannot serve as an analytical panacea The Chemical Shift • The Chemical shift makes NMR useful in Chemistry (they named it after us) • Arises from the electrons surrounding our nuclei, responding to a magnetic field. • Induced circulation of electrons, Lenz’s law; this circulation generates a small magnetic field opposed to H0 • The small negative field diminishes the H0 experienced by a nucleus. This differentiates sites, based on chemical nature • Effect grows directly proportional to H0 A Picture of this… How many signals do we Expect in an NMR Spectrum? • The Chemical shift implies that we see (potentially) a different signal for every different chemical environment. • Chemical environment here is the electronic structure (electrons, hybridization, charge, polarizability etc.) These are all things able to be predicted to some extent by theory. • What do we mean by “different”? (hint; symmetry is key!) Consider the Molecule of Interest for this Investigation… OH CH3 O O N H H H H H H H Because of the asymmetric carbon center at H, the other ring H are potentially all at different shifts (each is either on same, or opposite face from H) A Vector Picture Chemical shift is the ultimate precessional frequency of the vector component of M in the plane perpendicular to H0 H0(Z) This is in units of (radians)/sec At some time, has distinct angle and as a vector in x,y can be resolved into x, y components. H0(Z) t t X Y Precesses at a frequency X After a pulse… Y The receiver works by counting how many times this electric vector whizzes past in a unit of time Free Precession, Rotating Frames and the Chemical Shift •Our vector picture can help Now, more than one chemical shift wil move with just a difference from H0 Rotates at H0 MHz Stands Still! What if we could contrive to measure once every H0 seconds? Strobe effect Is The Rotating Frame Don’t have to distinguish 25000002 from 25000005 Hz, but 2 cf. 5 Imagine a “blinking eyeball”, (strobe effect) blinks at Larmor frequency…… “Practical” Theory • The real triumph of the shift theory is in its relationship to electronegativity and hybridization and easy prediction of trends based on qualitative notions from structural theory. • Withdrawing electron density diminishes the screening ability of the electron cloud and the absorbance of the nucleus goes to lower field. • Feeding in electron density sends nucleus to higher field. • “Moving” electrons have some real consequences on nearby chemical shifts. Anisotropic Shielding Near Electrons Cir culat ing Electr on Cloud responding to0 H I ncr eases t he t otal H f ield felt at H by ca. 1.5 ppm De s hi e ldi n g Re gi on S h ie l din g Re gion I nduced Curr ent I nduced Magnet ic Field Pronounced effect for aromatic, in line with e circulation Applied H 0Field Other Anisotropic Shielding Cones Nitriles, acetylenes Above, below plane shielded + isonitriles + + In plane deshielded + + + + Carbonyl, alkene C + + • Effects are ca. 2 ppm at most. Small pos + O Polarized effect • Most Significant when a nucleus is fixed in geometry with respect to the neighboring field. Best description is in L.M. Jackman, S. Sternhell, Applications of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry, Pergamon Press, (1969) ch.2 + + This chemical shift anisotropy is the basis for the separation, and the direction of the separation of signals in the Lab exercise on acetylproline Predict that the H signals for the protons will move to higher shift values when the C=O is pointed at them, compared with the other form H amide bond N O O CHR "cis" H OH N RCH O O "trans" OH Important point: one would have to identify which signals these are in the spectrum What are “Spin Systems”? A network of protons of which the members are mutually J-coupled to some (not necessarily all) of the nearby protons, via contiguous bonds. H CH2 OH H O H O H OH H H OH H H H H O H H S OCH3 H OH OH OH O OH H Breaks up the pathway H H How Do these Various Features show up in an NMR Spectrum? OH O H H H CH3 N Every different hydrogen in the molecule has (or is entitled to have) its own chemical shift value O H H The amount of this value is reflective of the chemical influence of nearby heteroatoms, electron deficency etc. The signals have extra splitting superimposed on them. This is coupling, caused by the neighboring Hs. Helps identify which H is which. H H Solvent (CD3OD) The red trace is called the integration (area under the curve). The amount of rise is proportional to the number of H that cause that particular signal. Chemical shift scale is x-axis. Units are Hz(shift)/Hz(spectrometer) and therefore ppm and dimensionless Integration of Signal areas in NMR Integration as the area under a curve Units are arbitrary, as the user defines the area scale. The real units would be mV x Hz but these numerically are unwieldy. Areas are taken relative to each other. Generally a recognized signal known to arise from one 1H is defined as 1.00. Can also add a weighed amount of an internal reference compound; assign an area to one of its signals, and compare the other signals to the reference. Integrating Spectra Inegral trails Area is given by the rise between the two level lines. Here we have used the software to reset the integral baseline between the two signals Signals Then, we use the software scale setting tool to define the peak at 8 ppm to be 3 units (H). The value for the other signal is then normalized and scaled so we know it is about 1% bigger. Repeated measurements can give us the precision (rsd) More on Integration Areas are proportional to molar ratios. Within a compound, if one signal is 3x another the signals represent atom counts of 1:3, e.g a CH, CH3. For mixtures, if we compare ratios of areas, these are the molar ratios Can convert to wgt% by multiplying by MW Must compare signals from same number of Hs or normalize to correct. Example Say we have a mixture and want to quantify two components by NMR. If we compare a CH3 group from compound A with a CH2 group from compound B, the comparison is not appropriate. (unless we know that those two signals are CH3 and CH2, and divide the areas by 3, and 2 respectively before making the comparison.) NMR of Mixtures •Potentially all the hydrogen atoms are equal to each other as “chromophores” •Compare with the situation of an HPLC analysis detected by means of a UV monitored flow-cell (monitoring a given wavelength). To interpret the areas of the HPLC peaks, one has to either know the response factors for all the compounds at that wavelength, (measure in a separate experiment) or assume that the responses are all identical on a molar basis (dubious at times). •NMR is good for mixture analysis also because you see everything that has hydrogen atoms. If you can locate signals that are not overlapped among the ingredients in the mixture, you can integrate, and obtain ratios of the molar amounts present. Practical Consequences of Relaxation times for Quantitative Interpretation Time constant that limits the repeat rate for NMR scans. Real concern in comparing disparate molecular sizes. Solvents vs. moderate size organics, common example A Recent Example from the Chem 314 Lab Plots are for varying the delay time between successive pulses. 35 30 Ibuprofen NMR Intensity Data is the integrated intensity of NMR signals from Ibuprofen vs an internal std of methylene chloride. 25 second delay 10secf ixup 5sec delay 25 20 15 10 5 0 100 200 300 400 500 600 700 mg Added Ibuprofen 800 900 Mixtures at equilibrium A B Keq C C ; AB where thebrackectedlettersrepresentthe molar concentrations of thereactingspecies. The NMR spectrum would likely show peaks from compound A, compound B and compound C. Some of these peaks could be overlapped. The proportions of these peaks for A, B, C would be related to how much the scientist put in the sample, and on the value of Keq The Mixture of Interest Here OH Every hydrogen in the compound gives a potentially different signal for the cis, and trans forms. For these, we can see and integrate the areas for the two forms as major, minor components. H H O H H H (Solvent) Acetyl CH3 CH3 O N H H H H Note here that the H, H happen to overlap. We cannot conveniently integrate these separately, or evaluate the major/minor ratio A Closer look at the fine structure… An Expansion of the NMR spectrum for the H region. Each of the compounds two forms shows its own hydrogen at a separate chemical shift. The individual peaks within these clusters are the fine structure due to couplings to nearby H’s Assessing Equilibria The integrated areas, normalized for the number of contributing signals, can be taken as proportional to the molarity. All the components are in the same volume of solution. To use the example for this lab exercise: cis trans trans Keq cis There are only two “compounds” in the mixture. Conc of each is proportional to int. area for each, normalized. Energies and dipole moments of NAcProline Conformers From ab initio (density functional at B3LYP/6-31G level of theory) calculations 6.36 debyes -553.8249 h 6.02 debyes -553.8072 h 5.74 debyes -553.5800 h 3.03 debyes -553.8136 h Systems Approaching Chemical Equilibrium A Collorary: Get time distribution curves at different Temperatures, e.g. Care must be taken that the time for the measurement is not significant w.r.t. the chemical time scale. A system of chemically related species may or may not be equilibrated. If you take a repeat spectrum are the ratios unchanged? Chemical Reactions and Kinetics and NMR Spectroscopy • NMR is a powerful technique for exploring reactions • Equilibrium and Kinetics are both accessible • In solution, we get total chemical picture (of NMR active atoms) • Can evaluate chemical exchange that is not accessible through other methods • Like any mechanistic study, requires controls, temperature regulation, careful integration, thoughtful interpretation Systems in Chemical Equilibrium NMR and Kinetics a great fit but… k1 A B k-1 (Hz) in kinetics is a reciprocal chemical lifetime (Hz) in NMR is a chemical shift A difference related to the 2 chemical environments B Spectra are affected when the 1/(chemical lifetime) becomes similar to the that separates the chemical shifts of the atoms in exchange. Important corollary: Since the chemical shifts of these two are what is observed, for the same process will vary with magnet strength. The same sample, same process, same NMR tube, same temperature can give two differentappearing spectra, at two different fields. Chemical process can be rotation, proton exchange, isomerization, rearrangement, dissociation or almost any reaction. Lifetime (sec) can be expressed as rate. The chemical and the NMR ’s different numbers!!! What is the Picture? Imagine a chemical species A, in chemical equilibrium with B, and that they have different NMR signals (can be proton, carbon phosphorus, etc.) Hz? Is k near in value to ? A B A and B are separated in the Spectrum by some number of Hz. What gives us the ability to see these as separated peaks? Hint, Hz is a reciprocal lifetime What is meant by “The NMR Time Scale”? •Imagine two signals that are chemically changing their identities. •They have chemical shifts, 1, 2 •These shifts are also separated by a given number of Hz; (=1-2) •Remember, that Hz has units of 1/sec. •The chemical shift difference in Hz can be compared to a “chemical lifetime” or its reciprocal the reaction rate constant k. k has units of 1/sec. •If the reaction rate k is faster than , we can only observe a signal at the average of the two chemical shifts. Intensity will be the sum. •We can address this experimentally by making k smaller (lower the temperature) or making bigger (use a higher field NMR magnet) •Practically, the relevant time scale for exchange here is 10s of msec. Take home message The NMR’s ability to see different signals for compounds that are in chemical exchange is limited. The limit is determined by the comparison of the rate (1/lifetime) for the chemistry, with the separation in Hz of the related signals.