Chapter 5
advertisement
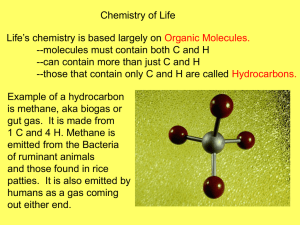
“Do Now” “W” of KWL What do you WANT to know about organic molecules and carbohydrates? – 5 things (with partner) • 5.1 Carbon is the main ingredient of organic molecules • 5.2 Carbohydrates provide fuel and building material 5.1 Carbon & Organic Molecules • Other than water, most molecules of a cell are carbon-based… “bio-molecules” – Why are most carbon based? • Carbon Skeletons!! • Besides bonding with other carbon atoms, carbon may also bond with atoms of other elements – 2 types of molecules • organic and inorganic So what… • The ability of carbon to form up to 4 bonds has made it essential for life’s functions • “Skeleton” backbone of any organic molecule (3) – Carbohydrates (sugars) (NRG) – Lipids (Fats, steroids, cholesterol) – Proteins (AA, hair, fur, muscles) (Storage) • Enzymes (Tools) 3 Main functions of carbon in Biology Organic Molecules • Organic molecules molecules containing carbon • Examples: sugars, fats, proteins, nucleic acids • Carbons bonding with other elements sometimes take on different names – carbon + hydrogen are hydrocarbons • Important fuels, storing fat in body – Oxygen and Nitrogen Inorganic Molecules • Inorganic molecules non-carbon molecules – Examples: • Ammonia (NH3), water (H20), baking soda (NaHCO3), carbon dioxide (Co2) Carbon & Functional Groups • Functional Group – “Functional groups have specific functions that they consistently do” – predictable way with other molecules – 4 Functional groups of carbon • Hydroxyl hydrophilic (attract water…molecules with this group will attract water) • Carbonyl • Carboxyl • Amino The Functional Group Structures • • C- OH (Single bond) C=OH (Double bond) • • C=O AND C-OH H • N H Monomers and Polymers Monomers are small molecular units, multiple monomers can be used to make polymers • Monomers = letters • Polymers = words • Biomolecules = sentences – (carbohydrates, lipids, proteins, nucleic acids) • Every living cells has thousands of different kinds of polymers • Varieties of polymers differ among individuals of the same species…even more some among different species Can we build it? YES we Can!! • Building and breaking polymers – EVERYTIME a monomer is added to a chain of polymers a water molecule is released! • DEHYDRATION reaction – b/c it involved removing (DE) water (HYDRATION) – EVERYTIME a polymer is broken down water is added to them • HYDROLYSIS reaction – b/c it involved added water (HYDRO) to break (Lysis) Quick Review 1. What is the difference between an organic and inorganic molecule? 2. What are the 4 functional groups? 3. Monomers make polymers…what are examples of polymer Biomolecules? 4. What kind of reaction occurs in the formation of a polymer? 5. What kind of reaction occurs in the break-down of a polymer? 5.2 Carbohydrates • Sugar = organic compounds • Sugars contain – 1 Carbon: 2 Hydrogen: 1 Oxygen • Sugar = carbon skeleton w/ ring shape – Monosaccharides – Disaccharides – Polysaccharides Monosaccharide • Simple Sugars (1 sugar unit) • Glucose, Fructose, Galactose – Sweet taste • Straight chains AND rings • Main fuel supply for cellular work – Cells breakdown glucose & extract stored NRG – What happens to glucose not immediately used? Disaccharides • Formed using dehydration reaction – 2 monosaccharides • Sucrose glucose linked to fructose • Once consumed it is broken down • Use right away or later on Polysaccharides What happens to glucose not immediately used? • Starch plant cells; starch chains coil up like phone chords; serves as sugar stockpile • Glycogen animal cells; more highly branched than starch; stored as granules' in liver and muscle cells • Cellulose plan cells; building material; protection, stiffness, cannot be digested by humans (fiber) All 3 made of glucose monomers Hydrophilic • All carbohydrates are hydrophilic – Due to hydroxyl groups • Mono & disaccharides easily dissolve • Cellulose doesn’t really Absorbs water well though 5.3 Lipids • Hydrophobic – Water avoiding molecules • Boundaries or chemical signals – Boundaries = fats; saturated or unsaturated – Chemical signals = steroids; cholesterol, estrogen, testosterone Fats • 3 carbon backbone glycerol • Attached to 3 fatty acids (hydrocarbons) • Saturated fat & Unsaturated Fat Saturated Fat • All the carbon atoms in the fatty acid chains form single bonds with each other – other bonds ALL contain hydrogen • Most animal fats, butter, lard • Solids at room temperature Unsaturated Fat • Less than the maximum number of hydrogen atoms in one or more of its fatty acid chains • Some carbons have double bonds causes kinks in chain • Fats in fruit, veggies, fish, olive oil • Liquid at room temp Steroids • Lipid molecule in which the carbon skeleton forms 4 fused rings • Steroids differ in the kinds & locations of functional groups attached • Classified as lipids because they are hydrophobic, differ greatly in structure • Chemical signals (estrogen & testosterone) pg 99 • Small variation with BIG difference in function – Cholesterol required in formation of other steroids… too much is bad Structure Where its found Examples Function Other Homework Quiz Friday on 5.1-5.3 Review Sheet Tomorrow! READ THE SECTIONS!! What did we talk about yesterday? • • • • • • • What is the name of compounds that contain carbon? What are the 3 types of carbohydrate “sugars?” What are 3 examples of polysaccharides? What RXN occurs when building a polymer/ breaking? What organism makes cellulose? What organism makes glycogen? What are the 4 Functional Groups? Quick Review • What does hydrophobic mean? • What is the structure of a lipid? • What is the difference between saturated fats and unsaturated fats? • What is the structure of a steroid? 5.4 Proteins • Molecular tool kit for cells • Functions of Proteins – Structure Polymer constructed from 20 AA monomers – Make up hair, fur, muscles, nutrient storage, antibodies & many other functions (enzymes) – Different structure causes different function Amino Acids (AA) • All AA have 3 things in common – Central carbon atom bonded to • Amino group • Carboxyl group • Hydrogen Atom • Unique side group – This is what differentiates the 20 AA from each other Building Proteins • Create proteins by linking AA together into chains = polypeptide chain (multiple) – Done using dehydration reaction b/w amino group of one and the carboxyl of another – AA are “letters,” only 20 – Each proteins has a unique sequence of AA Protein Shape • Proteins cannot function as a single chain • Functional proteins consist of one or more polypeptide chains precisely twisted, folded, and coiled • Influence shape: – environment (water…yay or nay), temperature, pH • Unfavorable environment denaturation • Loose shape = loose function 5.5 Enzymes • Are proteins that catalyze chemical reactions – Speeds up chemical reactions by lowering the level of activation energy • NRG needed to start a chemical a RXN • Allow for reactions to occur at normal temperature • PAGE 103 • Each cell is like a mini chemical factory needed to carry out different reactions Activation Energy • Start-up energy needed to weaken chemical bonds • with catalysts (enzymes) • Provide a way for reactions to occur at the cells normal temperature • Enzymes lower the amount of energy required for a chemical reaction to occur • Enzymes are specific “puzzle pieces” Enzymes • Are chemical reaction specific – Puzzle piece fit • Reactant is substrate – What is being reacted on • Enzyme hold it in place – At active site, changes slightly • Can also speed RXNs by accepting 2 substrates adjacently – Breakdown larger molecules more easily pH, temperature affect enzyme too.. they ARE proteins Reflection Activity • Complete 5.5 Concept Checks Chapter Objectives • • • • • • Identify carbon skeletons and functional groups Relate monomers and polymers Process of building and breaking polymers Identify 3 polysaccharides and their functions General characteristics of lipids Structure and Function of sugar, fats, steroids, proteins, AA • Factors that influence protein shape • How enzymes affect activation NRG • How enzyme shape affects functions