Biomolecules - Fall River Public Schools
advertisement

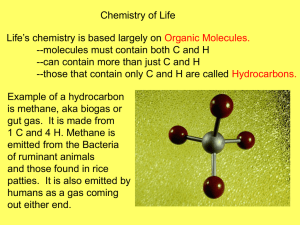
Biomolecules Biological Molecules “Molecules of Life” • Also called Organic molecules Biological Molecules • Biological molecules are large molecules found in all living things 4 Types of Biomolecules • • • • 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids CHNOPS These molecules are • All these molecules made of the elements: contain the element Carbon • Carbon • Hydrogen • Nitrogen • Oxygen • Phosphorus • Sulfur All living things contain Carbon Carbohydrates A fancy way of saying sugar! Functions 1. Short-term energy storage 2. Gives organisms Shape/Structure: Example1: Exoskeleton of crabs and beetles Example: 2 Cellulose: plant cell walls Examples: Carbs are in plants! • Glucose = sugar in plants • Fructose = in fruit • Lactose = in milk • Sucrose = table sugar Words for sugars end in “ose” The subunit of a carbohydrate is a Monosaccharide Mono = 1 Saccharide = sugar Monosaccharide = 1 sugar molecule • Disaccharide = 2 sugars Starches are polysaccharides Polysaccharide = many sugars More names for Carbohydrates Structure • Carbohydrates are ring-shaped molecules Elements • The elements that bond to make carbohydrates are: • Carbon • Hydrogen • Oxygen Lipids= Fats Function • Long term energy storage • Common names= fat, oil • Elements= C,H,O Lipid facts • Large , organic molecules • Won’t dissolve in water • Fats store more energy than carbs because they have many carbonhydrogen bonds • Can be “saturated” or “unsaturated" Saturated and Unsaturated fats • Saturated= solid at room temperature, Raise “bad” (LDL) cholesterol levels Ex= animal products, coconut • Unsaturated=liquid at room temperature, Raise “good” (HDL) cholesterol Ex= olive oil, avocado, almonds More Examples • Lard • Steroids: examples Cholesterol & testosterone • Waxes (like earwax!) • Phospholipids: these make up your cell membrane Phospholipids Lipids • Subunit= fatty Acids • Shape= chains Proteins 2 Main Functions Examples= 1. Form structures, like muscle Meat, muscle, enzymes 2.Act as Enzymes, which speed chemical reactions Elements= C,H,O,N,S Proteins’ subunit is amino acids • Proteins are one of the most diverse biomolecules, having lots of different shapes • They are composed of 20 different types of amino acids • Amino acids have an amino group (-NH3) and a carboxyl group (-COOH) More Functions of Proteins • Control rates of reactions & regulate cell processes • Form bones & muscles • Transport substances in & out of cells • Help fight disease. ENZYMES are Proteins! Enzymes are important proteins that speed up the chemical reactions in your body • Because they help these reactions happen, they are called catalysts Ex. The enzyme amylase helps break down carbohydrates into sugar when you chew How Enzymes Work • Every reaction needs energy to get started; this is called activation energy • Enzymes decrease the amount of energy needed to get these reactions going • Different reactions in your body need different enzymes • Without the correct enzyme available, your body cannot function properly Enzyme Action • How well enzymes work depend on 3 criteria: temperature, pH & concentration 1. Temperature: enzymes in your body work best at normal body temperature 2. pH: Different enzymes work best at different pH levels 3. Concentration: in general, the higher the concentration, the better the enzyme will work at speeding up the reaction. pH Scale Nucleic Acids • Elements: CHNOP • Functions: Store and transmit genetic information • 2 Kinds to remember 1. DNA= deoxyribonucleic acid 2. RNA= ribonucleic acid Nucleic acids- shape & subunit Shape of DNA= double helix Shape of RNA= single strand Subunit= nucleotide 3 parts: 1. Sugar 2. Phosphate 3. Nitrogen-containing base • DNA has 4 Types of bases= A,T,C,G (adenine, thymine, cytosine & guanine) Compare DNA and RNA DNA structure= double helix RNA structure= single strand