SCH3U 4.1:4.5 Law of Definite Proportions:Percent Compositi

Quantities in Chemical
Reactions
(4.1/4.5) Proportions in
Compounds and Percentage
Composition
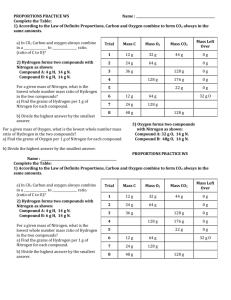
Law of Definite Proportions
a specific compound always contains the same elements in definite proportions by mass, regardless of how it is synthesized
compounds with the same mass proportions must be the same compound
the proportions are found by calculating the percent by mass .
Percent by Mass
(percentage composition)
based on the law of conservation of mass
MASS compd
= sum of MASSES elements
% by mass = MASS element x 100%
MASS compd
Percent by Mass
example: H
2
O
made up of 2 atoms of hydrogen and 1 atom of oxygen
to find percent by mass of each element:
H= (mass H / mass of water) x 100%
O= (mass O / mass of water) x 100%
Percent by Mass of H
2
O
Mass % of H = mass of H (X2) X 100 mass of H
2
O
= 1.01u X 2 X 100
1.01u X 2 + 16.00
= 2.02u
X 100
18.02u
= 11.2%
Mass % of O = 100% - 11.2%
= 88.8%
Practice Problems
Q: A 78.0 g sample of an unknown compound contains 12.4g of hydrogen. What is the percent by mass of hydrogen in the compound?
A: % Mass H = mass H x 100% mass comp
= 12.4g x 100%
78.0g
= 15.9%
Practice Problems
Q: How many grams of oxygen can be produced from the decomposition of
100.0 g of KClO
3
?
A: % mass O = mass O x 100% mass KClO
3
= 3(16.00)u x
100%
[39.10+35.45+3(16.00)]u
= 39.17%
Practice Problems
Q: How many grams of oxygen can be produced from the decomposition of
100.0 g of KClO
3
?
A (continued): mass O = %O x mass KClO
3
= 0.3917 X 100.0g
= 39.17g
Practice Problems
Q: Two unknown compounds are tested.
Compound 1 contains 15.0g of hydrogen and 120.0g oxygen .
Compound 2 contains 2.0g of hydrogen and 32.0g oxygen . Are the compounds the same?
HINT!! If % Masses are equal , then they are the same
A: Compd 1-
%H = [15.0 / (15.0+120.0)] x 100%
= 11.1%
%O = [120.0 / (15.0+120.0)] x 100%
= 88.9%
Compd 2-
%H = [2.0 / (2.0+32.0)] x 100%
= 5.9%
%O = [32.0 / (2.0+32.0)] x 100%
= 94.1%
NOT THE SAME COMPOUNDS
Homework
Read pg. 160 – 162 & pg. 178 - 184
Finish “Percent Composition
Worksheet ”
pg. 184 “Section 4.5 Questions” #3 - 5