Solution Concentrations
advertisement

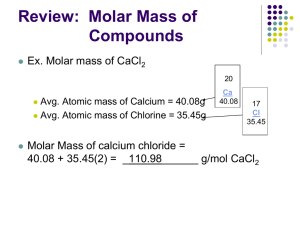
Molar Relationships Part 4: Molar Relationships • The mole and molar calculations • Stoichiometry • Solution Concentrations • Acid/Base Theory You will need a calculator and periodic table to complete this section. The Mole and Mole Calculations One mole = 6.02 x 1023 representative particles One mole = 22.4 Liters of gas at 0°C and one atmosphere of pressure One mole = the atomic mass listed on the periodic table. For example: one mole of Helium contains 6.02 x 1023 atoms of Helium and it has a mass of 4.00260 grams. At 0°C and one atmosphere of pressure, it would occupy 22.4 Liters. Sample problem: How many liters would 2.0 moles of Neon occupy? Answer: 2.0 moles Ne x 22.4 Liters Ne = 44.8 Liters Ne 1.0 moles Ne Sample problem: How many moles are in 15.2 grams of Lithium? Answer: 15.2 g Li x 1 mole Li = 2.19 mole Li 6.941 g Li The Mole and Mole Calculations One mole = 6.02 x 1023 representative particles One mole = 22.4 Liters of gas at 0°C and one atmosphere of pressure One mole = the atomic mass listed on the periodic table. Sample problem: How many liters would 14 grams of Helium occupy? Answer: 14 g He x 1 mole He x 22.4 L He = 78 Liters He 4.0026 g He 1 mole He The Mole and Mole Calculations One mole = 6.02 x 1023 representative particles One mole = 22.4 Liters of gas at 0°C and one atmosphere of pressure One mole = the atomic mass listed on the periodic table. You try one: What is the mass of 9.0 Liters of Argon gas at 0°C and one atmosphere of pressure? The Mole and Mole Calculations One mole = 6.02 x 1023 representative particles One mole = 22.4 Liters of gas at 0°C and one atmosphere of pressure One mole = the atomic mass listed on the periodic table. You try one: What is the mass of 9.0 Liters of Argon gas at 0°C and one atmosphere of pressure? 9.0 L Ar x 1 mol Ar x 22.4 L Ar 39.948 g Ar = 16 g Ar 1 mole Ar The Mole and Mole Calculations The molar mass = the sum of all the atomic masses. Example Ca(NO3)2 = 40.08 + 2(14.01) + 6(16.00) = 164.10 grams You try one: What is the gram formula mass (molar mass) of Mg3(PO4)2? The Mole and Mole Calculations The molar mass = the sum of all the atomic masses. Example Ca(NO3)2 = 40.08 + 2(14.01) + 6(16.00) = 164.10 grams You try one: What is the gram formula mass (molar mass) of Mg3(PO4)2? 3(24.305) + 2(30.97376) + 8(15.9994) = 262.86 grams The Mole and Mole Calculations The molar mass = the sum of all the atomic masses. Example Ca(NO3)2 = 40.08 + 2(14.01) + 6(16.00) = 164.10 grams You try one: What is the gram formula mass (molar mass) of Mg3(PO4)2? 3(24.305) + 2(30.97376) + 8(15.9994) = 262.86 grams What is the percent Magnesium in Mg3(PO4)2? Answer: 3(24.305) x 100 = 27.7% 262.86 You try one: What is the percent Lithium in Li2SiO3? molar mass = 2(6.941) + 28.0855 + 3(15.9994) = 89.9657 g % Li = 2(6.941) x 100 = 15.4% 89.9657 A Brief Return to Empirical Formulas Empirical Formulas are the reduced form of Molecular formulas. For example: The empirical formula for C5H10 is CH2. A favorite exam question: What is the empirical formula of a compound that contains 30% Nitrogen and 70% Oxygen? a) N2O b) NO2 c) N2O5 d) NO This is really a percent composition problem. Figure out which compound contains 30% nitrogen. The Mole and Mole Calculations At Standard Temperature and Pressure (STP) 1 mole of gas = 22.4 L You can use this to calculate the density of a gas in g/Liter at STP. Example: What is the density of CO2 gas at STP? The molar mass of CO2 = 12.0111 + 2(15.9994) = 44.0099 g Density = mass/volume = 44.0099 g/22.4 L = 1.96 g/L The Mole and Mole Calculations At Standard Temperature and Pressure (STP) 1 mole of gas = 22.4 L You can use this to calculate the density of a gas in g/Liter at STP. Example: What is the density of CO2 gas at STP? The molar mass of CO2 = 12.0111 + 2(15.9994) = 44.0099 g Density = mass/volume = 44.0099 g/22.4 L = 1.96 g/L You try one: What is the density of Cl2 gas at STP? The Mole and Mole Calculations At Standard Temperature and Pressure (STP) 1 mole of gas = 22.4 L You can use this to calculate the density of a gas in g/Liter at STP. Example: What is the density of CO2 gas at STP? The molar mass of CO2 = 12.0111 + 2(15.9994) = 44.0099 g Density = mass/volume = 44.0099 g/22.4 L = 1.96 g/L You try one: What is the density of Cl2 gas at STP? Answer: molar mass = 2(35.453) = 70.906 g 70.906 g/22.4 L = 3.165 g/L Stoichiometry For reaction calculations, the molar ratio is used. Example: How many moles of nitrogen will react with 9 moles of hydrogen to produce ammonia according to this equation? 2N2(g) +3 H2(g) → 3NH3(g) Given: 9 moles H2, Find moles N2 9 mol H2 x 2 mol N2 = 6 mol N2 3 mol H2 Mole ratio Stoichiometry For reaction calculations, the molar ratio is used. Example 2: How many grams of nitrogen are needed to react with 2.0 grams of hydrogen using this equation? 2N2(g) +3 H2(g) → 3NH3(g) Given: 2.0 grams H2, Find grams N2 2.0 g H2 x 1 mol H2 2.016g H2 x 2 mol N2 x 28.014 g N2 3 mol H2 1 mol N2 = 18.53 g N2 Solution Concentrations Calculating molarity: Memorize this equation: Molarity = moles/liters or M = mol/L Memorize conversion factor: 1000 mL = 1 L Some example of using this equation: Example 1: the molarity of 2.0 moles of HCl in a 0.50 L solution of water is: molarity = 2.0 mole HCl/0.50 L = 4.0 Molar or 4 M Example 2: The molarity of 0.40 moles of HCl in a 300. mL L solution of water is: molarity = 0.40 moles HCl/0.300. L = = 1.3 M Solution Concentrations Example 3: The molarity of 72.9 g of HCl in 5.0 liters of aqueous solution is: Answer: first calculate the moles of HCl 72.9 g HCl x 1 mol HCl = 2.00 mol HCl 36.46 g HCl Then calculate molarity of solution: 2.00 mol HCl/5.0 L = 0.40 M HCl Solution Concentrations You try one: What is the molarity of 1.2 grams LiF in a 50. mL aqeous solution? Answer: first calculate the moles of LiF 1.2 g LiF x 1 mol LiF = 0.046 mol LiF 25.94 g LiF Then calculate molarity of solution (remember convert mL to Liters): 0.046 mol LiF/0.050 L = 0.95 M LiF Solution Concentrations Diluting concentrated solutions Memorize: M1V1 = M2V2 •M1 and V1 are the beginning molarities and volumes •M2 and V2 are the ending molarities and volumes •V1 and V2 can be in Liters or mLs, but must be the same units for both Example: What is the molarity of a 10. mL sample of 2.0 M aqueous HCl diluted to 40. mL Answer: (2.0)(10.) = (M2)(40.) so M2 = 0.5 Molar HCl Solution Concentrations Diluting concentrated solutions Memorize: M1V1 = M2V2 •M1 and V1 are the beginning molarities and volumes •M2 and V2 are the ending molarities and volumes •V1 and V2 can be in Liters or mLs, but must be the same units for both You try one: How many milliliters of 6.0 Molar HCl are required to prepare 240 mL of 2.0 Molar HCl? Answer: (6.0)(V1) = (2.0)(240) so V1 = 80. mL HCl Chemical Equilibrium Exothermic and Endothermic Reactions Catalysts lower the Activation energy barrier, making reactions faster. 100 100 Joules > Endothermic reactions absorb heat Joules > Exothermic reactions release heat 0 0 rxn progress > A + B = AB + heat rxn progress > A + B + heat = AB Acid/Base Theory Acids and Bases Generic formula for acids = HX (HCl, HNO3, H2SO4) Generic formula for bases = MOH where M is any metal (NaOH, KOH, Ca(OH)2 Ammonia, NH3, is also a base. Acid solutions have a pH less than 7 Basic solutions have a pH more than 7 Arrhenius acids: sour Taste _______ turn litmus paper red. SAFETY NOTES Arrhenius bases Taste _______ bitter slippery Feel __________ Turn litmus paper blue. If you spill acid or base on yourself, rinse with lots of water. Always add acid to water when diluting Acid/Base Theory What is pH? pH indicates the hydrogen ion molarity [H+] in a solution pH = -log[H+] pOH indicates the hydroxide ion molarity [OH-] in a solution. pOH = -log[OH-] Example: A 1.0 x 10-3 molar solution of HCl would have a pH of ___ 3 4 Example: A 1.0 x 10-4 molar solution of KOH would have a pOH of ___ Memorize: pH + pOH = 14. 6 Example: A solution with a pH of 8 will have a pOH of: ____.