Lecture Slides for Bonding and Acids/Bases
advertisement

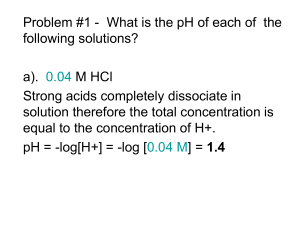
CH339K Bonding, Water, Acids, Bases, and Buffers Bonding • • • • • • Covalent Ionic Dipole Interactions Van der Waals Forces Hydrogen Bonds Hydrophobic Interactions Covalent Bonds • Electrons form new orbitals around multiple atomic nuclei • Bond energy results from electrostatic force between redefined electron cloud and nuclei • Strong – typically 150 – 450 kJ/mol Common Covalent Bond Numbers For Biochemically Significant Elements Atom Bond Number* C 4 H 1 O 2 N 3 P 3,5 S 2,4,6 *the bond numbers illustrated are typical of biological systems and should not be considered set in stone. i.e. Don’t depend on this for your Inorganic class! Ionic Interactions • Biomolecules frequently have large numbers of charged groups • Charge-charge interactions stabilize intra- and intermolecular structures Ribbon representation (A) and surface charge distribution of (B) of the lowest-energy solution structure of the SARS-CoV X4 ectodomain. Red and blue surfaces represent negative and positive electrostatic potentials, respectively. Ionic Interactions • Energy from non-directional electrostatic force between ions • Coulomb’s Law: q1q 2 Fk 2 er r q1q 2 U Fr dr k er Fr • Energy drops off as function of distance between charges (i.e. operates over long ranges) e is the dielectric constant of the medium – k is the Permittivity of the Vacuum – sort of an absolute dielectric constant to which other dielectric constants relate. • Ionic interactions tend to be quite strong. Some enchanted evening, you may see a stranger; you may see a stranger across a … …empty room WEAK Dielectric …crowded room STRONG Dielectric Common Dielectric Constants Name water methanol ethanol 1-propanol 1-butanol formic acid acetic acid acetone hexane benzene dielectric constant 80 33 24.3 20.1 17.8 58 6.15 20.7 2.02 2.28 Dipoles • Fixed dipoles – Molecules with asymmetric charge distributions form dipoles O + H3N CH2 C O - • Induced Dipoles – One dipole can induce a charge in an adjacent molecule O + H3N CH2 - C O Dipole moments m = q·x – Where m is the dipole moment – q is the charge – x is the distance between the charges The larger the dipole moment, the more polar the molecule. van der Waals Interactions • Technically, all induced dipole interactions are van der Waals interactions • Biochemists usually mean induced dipole-induced dipole (London Dispersion) forces • Any atom will have an uneven distribution of charge at any given instant Van der Waals (cont.) • That temporary dipole will induce a dipole in adjacent atoms • This results in a net attractive force between atoms • Force is weak - .5 to 2 kJ/mol • Net biochemical effect – molecules that FIT together STICK together. Van der Waals (cont.) • If you live in Central Texas, you see van der Waals forces in action every summer night: Artificial Geckos Climb building Crawl through window Find target Detonate Real gecko toe hair (Courtesy of Geico) Synthetic gecko toe hair Protein – DNA Interaction Charge Interaction Energies and Distance Van der Waals Forces (cont.) London Dispersion Forces cause particles to come together… Until they get too close. Atomic Radii The Lennard-Jones potential describes the interaction between a pair of neutral atoms: 5 1 1 U α 12 6 r r Energy (arbitrary units) 4 3 2 1 0 0.5 1 1.5 -1 -2 -3 Electron Cloud Repulsion London Dispersion Force Radius (Å) 2 Multiple molecular contacts can mediate binding hexokinase Energy of van der Waals contacts can subsidize conformational changes in molecules. Hydrogen Bonds • Hydrogen Bonds form between – A hydrogen covalently bound to an electronegative atom – Another electronegative atom Hydrogen Bonds (cont.) • The group to which the hydrogen is covalently bound is the donor. • The other group is the acceptor. • Donors: – -OH, -NH2, -SH (lesser donor) • Acceptors – -N:, =O:, -O: H Hydrogen Bonds (cont.) • Bond length is less than vdW contact distances • distance between the nuclei of the hydrogen bond acceptor and the hydrogen itself can be as short as 1.8-1.9 Å, well below the sum of the atomic radii (e.g. 1.2Å for hydrogen and ~1.5Å for oxygen and nitrogen) Hydrogen Bonds (cont.) • Intermediate strength: 5 – 30 kJ/mol • Hydrogen bonds are not just electrostatic – partially covalent • Therefore, they are directional a-helix: An internal protein structure mediated by Hydrogen Bonds (amide hydrogens to carbonyl oxygens) Binding from both H-Bonds and vdW Contacts DNA Double Helix EcoR1 – a DNA-cleaving Protein Recap of Bond Energies (typical) STRENGTH (kcal/mole) BOND TYPE LENGTH (nm) IN VACUUM IN WATER Covalent 0.15 90 90 Noncovalent: ionic 0.25 80 3 hydrogen 0.30 4 1 van der Waals attraction (per atom) 0.35 0.1 0.1 Copyright © 2002 Bruce Alberts, Dennis Bray, Julian Lewis, Martin Raff, Keith Roberts, and James D. Watson Water Structure Water Forms Clusters in Solution Dissolving nonpolar molecules • Solvating a nonpolar molecule imposes order on the surrounding water - (DS < 0) Clathrate cage of ordered water Hydrophobic interactions Solvating a non-polar material in water decreases entropy – forces water into an ordered structure Minimal energy is when water is least ordered – the more you can pack nonpolar materials, the less surface area exposed to solvent. Hydrophobic Effect Recap – bonding in biomolecules Aka Salt Links Ice Floats Water • Water – – – – – – – Has a high specific heat Has a high heat of vaporization Is an excellent solvent for polar materials Is a powerful dielectric Readily forms hydrogen bonds Has a strong surface tension Is less dense when it freezes (i.e. ice floats) Acids and Bases • Definitions – Arrhenius • Acids are substances which produce an excess of H+ ions in water (HCl) • Bases are substances which produce an excess of OH- in water (NaOH) – Bronsted-Lowry • Acids are substances which can donate a proton in a chemical reaction. (HF) • Bases are substances which can accept a proton in a chemical reaction (NH3) – Lewis • Acids are electron - pair acceptors.(BF3) • Bases are electron - pair donors (CaO) Conjugate Pairs • Every acid has its conjugate base • Every base has its conjugate acid Conjugate Acid Conjugate Base H3C - COOH H3C-COO- NH4+ NH3 Water can act as both an acid and a base. Ionization of Water The extra proton of a hydronium ion is not confined to a single water molecule. Ionization of Water Kw = [H+] [OH-] = 10-14 (at 25 C, 1 atm) Kw is small, so water has a very low amount of ionization. Kw is temperature dependent! Kw = 10-13 at 60 C. [H+] is a measure of acidity. However, it ranges over many orders of magnitude. For ease of arithmetic, we generally refer to pH pH = -log[H+] (for our purposes) Typical pH Values Substance pH Stomach acid 1.5 - 2.5 Coca-cola 2.5 Human saliva 6.5 Human blood 7.5 Human urine 5-8 Oven cleaner 14 Acids and Bases • A Strong acid is one that dissociates completely in water; a weak acid is one that doesn’t. – Hydrochloric, Hydroiodic, Hydrobromic, Nitric, Sulfuric, Perchloric • All biochemically significant acids and bases are weak – i.e. they don’t dissociate completely (except for HCl – stomach acid) Acids and Bases The strength of a weak acid can be described by the Ka (ion product) and the pKa. The lower the pKa, the stronger the acid. Typical Ka’s and pKa’s Acid Ka pKa Acetic 1.8 x 10 Formic 1.7 x 10 Benzoic 6.5 x 10 Carbonic 4.3 x 10 Imidazole 2.8 x 10 Phenol 1.3 x 10 -5 -4 -5 -7 -7 -10 4.76 3.75 4.19 6.37 6.55 9.89 pH for Strong Acids • Since a strong acid dissociates completely: pH = -log([Acid]) • For a 0.1 M (100 mM) solution of HCl: pH = -log(0.1) = 1 • Well, that was difficult… pH for Weak Acids • Depends on the Ka • What’s the pH of a 100 mM solution of Acetic Acid? 2 [H ][H CCOO ] [H ] 5 3 K a 1.810 [H3CCOOH] (0.1M- [H ]) [H ]2 Ka [H ] (0.1)[Ka ] 0 K a Ka 4(0.1)Ka 2 [H ] 2 [H+] = 0.00134 M QuadraticFormula: b b 2 4ac x 2a Shortcut • The quadratic solution is a pain, but we can approximate: [H ] K a [HAc] K a (0.1) [H+] = 0.00134 M • Accurate as long as acid < 5% dissociated Titrating a Strong Acid 10 ml of an HCl sln. Titrate with 0.5 M NaOH OH- + H+ → H2O Takes 8.5 ml NaOH to bring solution to neutrality 14 12 10 8 pH • • • • Titration of Strong Acid 6 4 V1C1 V2 C 2 or C1 C1 V2 C 2 V1 0.0085 L 0.5 M 0.425 M 0.010 L 2 0 0 5 10 NaOH added (m l) 15 20 Titrating a Weak Acid Titration of Weak Acid 8 7 6 5 pH • Titrating .1 M HAc • Initial pH is 2.88 instead of 1 • Little change until large amounts of NaOH have been added • Buffering effect • Caused by equilibrium that exists between a weak acid and conjugate base. 4 3 2 1 0 0 5 10 15 NaOH added (m l) 20 25 Henderson-Hasselbalch Equation [H ][A ] Ka [HA] [H ][A ] log pKa [HA] log[H ] log[A ] log[HA] pKa pH pKa log[A ] log[HA] [A ] pH pKa log [HA] Henderson-Hasselbalch Equation Predicting pH • Let’s make 1 liter of a solution that is 0.1 M in acetic acid ( pKa = 4.76 ) and 0.3 M in sodium acetate. [A ] pH pKa log [HA] 0.3 pH 4.76 log 0.1 pH 5.24 Buffering Effect • pH depends on pKa and the ratio of conjugate base to acid. • Addition of significant amounts of acid or base changes the ratio of conjugate base to conjugate acid • pH changes as the log of that ratio • Result is resistance to pH change in a buffered solution Factors impacting pKa: Ionic Strength The ionic strength of a system is the sum of contributions from all ions present: J Ci Z where 2 i i Ci is the concentration of ion I, Zi is the charge on ion I Factors impacting pKa: Ionic Strength Example: Phosphoric Acid has 3 pKa’s H3PO4 ⇄ H+ + H2PO4- ⇄ 2H+ + HPO4-2 ⇄ 3H+ + PO4-3 pKa1 pKa2 pKa3 pKa2 = 7.2 at ionic strength J = 0 pKa2 = 6.86 at physiological ionic strengths (Physiological saline is 0.91% NaCl. Calculation of J is left as an exercise for the student) Factors impacting pKa: Temperature pKa can (i.e. does) vary with temperature Example: one of the most common biochemical buffers is Tris (tris(hydroxymethyl)aminomethane) Tris is a good buffer at near- physiological pHs, is biologically pretty inert, and is (relatively) inexpensive. BUT Tris has a large thermal coefficient: 0.031 units/oC At 25o C, At 0o C pKa = 8.30 pKa = 7.77 A Physiological Example: Blood pH • Blood pH is maintained at ~7.4 – pH below 7.35 is acidosis – pH above 7.45 is alkalosis • pH < 7.0 or > 7.8 is generally fatal Blood pH Control Blood pH is regulated by four buffer systems: • • • • Carbonate H2CO3 ⇄ H+ + HCO3Phosphate H2PO4- ⇄ H+ + HPO4-2 Plasma Proteins Hemoglobin pKa = 6.1 pKa = 6.9 The primary system, carbonate, has 3 interlocking equilibria: CO2(g) ⇄ CO2(aq) + H2O ⇄ H2CO3 ⇄ H+ + HCO3Excess H+ or HCO3- drives the equilibrium to the left Excess H2CO3 drives the equilibrium to the right Blood pH Control CO2(g) ⇄ CO2(aq) + H2O ⇄ H2CO3 ⇄ H+ + HCO3• Diseases that effect the level of [HCO3-] are metabolic effects, due to changes in cellular metabolism. • Diseases that change [H2CO3] are respiratory effects; the lungs control the exchange of CO2, and therefore the concentration of H2CO3 . Blood pH Control CO2(g) ⇄ CO2(aq) + H2O ⇄ H2CO3 ⇄ H+ + HCO3- Metabolic Acidosis: • Diseases such as diabetes or diarrhea result in an excess of H+ in the tissues. • [HCO3-] goes DOWN (equilibrium pushed to left) • Blood pH goes DOWN. (equilibrium to left; higher carbonic acid, lower bicarbonate) Blood pH Control CO2(g) ⇄ CO2(aq) + H2O ⇄ H2CO3 ⇄ H+ + HCO3- Metabolic Alkalosis: • Vomiting (intoxication, gastrointestinal illnesses) causes loss of H+. • [HCO3-] goes UP (equilibrium pulled to right) • Blood pH goes UP. (equilibrium to right; lowerer carbonic acid, higher bicarbonate) Blood pH Control CO2(g) ⇄ CO2(aq) + H2O ⇄ H2CO3 ⇄ H+ + HCO3- Respiratory Acidosis: • In conditions like emphysema, pneumonia, your lungs do not work effectively to clear CO2. • [H2CO3] goes UP (driven by carbon dioxide build-up.) • Blood pH goes DOWN (as carbonic acid accumulates.) Blood pH Control CO2(g) ⇄ CO2(aq) + H2O ⇄ H2CO3 ⇄ H+ + HCO3- Respiratory Alkalosis: • When you hyperventilate or become hysterical, you blow off lots of CO2. • [H2CO3] goes DOWN (since its being withdrawn as CO2.) • Blood pH goes UP (less carbonic acid.) A Practical Buffer Problem Benzoic acid is a weak carboxylic acid that is reasonably soluble in water (3.4 g/l). – Molecular Weight: – pKa 122.12 g/mol 4.21 HO O I wish to make 4 liters of 10 mM Sodium Benzoate buffer, pH 5.0. I have solid benzoic acid in a jar, a stock solution of 5 M Sodium Hydroxide (NaOH), a 4 liter graduated cylinder and all the deionized, distilled water I can use. How do I make the buffer? Practical Buffer Problem (cont.) • Step 1: Okay, how much benzoic acid do I need? Since benzoate will be the buffering ion, I want my solution to be 10 mM in total benzoate. Solid benzoic acid is my only source of benzoate, so I need to add 10 mM worth: 10mM = 0.01 mol/l 0.01mol/l × 4 l × 122.12 g/mol = 4.88 g Practical Buffer Problem (cont.) Step 2: How do I get it to the right pH? • The conjugate base of benzoic acid is benzoate anion. • Addition of a strong base (like NaOH) to benzoic acid converts it to benzoate. • The pH of the solution depends on the ratio of conjugate base to conjugate acid as determined by the Henderson-Hasselbach equation. • How much benzoic acid to I have to convert to benzoate base to give me the desired ratio of conjugate base to conjugate acid? Practical Buffer Problem (cont.) [conjugate base] pH = pKa + log [conjugate acid] [benzoate] 5.0 = 4.21 + log [benzoic acid] [benzoate] 0.79 = log [benzoic acid] 6.17 = [benzoate] [benzoic acid] 6.17 [benzoic acid] = [benzoate] Rats! 1 equation with 2 unknowns… But wait! That’s not all! We also know that total benzoate is 10 mM [benzoate] + [benzoic acid] = 10 mM 6.17 [benzoic acid] + [benzoic acid] = 10 mM 7.17 [benzoic acid] = 10 mM [benzoic acid] = 1.39 mM [benzoate] = 10 mM - 1.39 mM = 8.61 mM We need to convert 8.61 mM benzoic acid to the conjugate base, benzoate. To convert 8.61 mM benzoic acid to 8.61 mM benzoate, we need to add 8.61 mM (.00861 M) NaOH 0.00861 mol/l desired 4 l total volume = 0.00689 l 5 mol/l stock So: add 4.88 g of Benzoic Acid, 6.89 ml of 5M NaOH, and enough H2O to make 4 liters. What you ought to be able to do after this agony • Know the different “bond” types involved in biomolecular interactions • Know their relative strengths (i.e. bond energies) • Be able to calculate ionic interaction energies • Understand what the van der Waals radius is • Be able to recognize H-bond acceptors and donors • Understand hydrophobic interactions • Be able to identify conjugate acids and bases • Understand the ideas of pH, Ka, and pKa • Be able to calculate pH and to calculate how to make buffers