CH 3 COO
advertisement

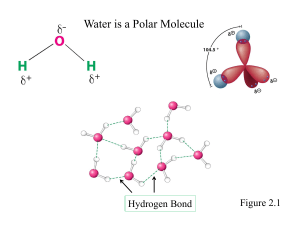
Basic Biochemistry CHE 242 MTWR 11:00 am – 12:35 pm Julian Hall 225 Dr. Jon A. Friesen Office: 318 Science Laboratory Building phone: (43)8-7850 email: jfriese@ilstu.edu What Do Biochemists Study? The periodic table of the elements Figure 1.1 The periodic table of the elements Figure 1.1 Bulk elements 97% The periodic table of the elements Figure 1.1 Essential ions The periodic table of the elements Figure 1.1 Trace elements Organic compounds in biochemistry Figure 1.2 Functional groups in biochemistry Figure 1.2 Linkages in biochemical compounds Figure 1.2 Types of molecules in biochemistry 1. Proteins Are composed of twenty different kinds of monomeric units, the amino acids. 2. Polysaccharides (sugar) Are constructed of monomeric units called monosaccharides. Also called carbohydrates. 3. Nucleic acids (DNA and RNA) Are synthesized from monomeric units called nucleotides. 4. Lipids (Fat) Water insoluble molecule containing fatty acids. Used for membrane structure and energy storage. Energy Flow Page 11 Prokaryotic Cells Figure 1.16 Eukaryotic Cells Figure 1.18 Water, Water Everywhere Water is a Polar Molecule Figure 2.1 Polarity of Small Molecules Figure 2.2 Hydrogen Bonding Between Two Water Molecules Figure 2.3 Water Can Form Up To Four Hydrogen Bonds Figure 2.4 Water Molecules Form a Hexagonal Lattice in Ice Figure 2.5 Sodium Chloride (NaCl) crystal Figure 2.6 Ionic and Polar Substances Dissolve in Water Example: Dissolution of Sodium Chloride in water Figure 2.6 Glucose, a sugar, contains polar groups, and is soluble in water Nonpolar substances are relatively insoluble in water Noncovalent interactions in biomolecules 1. Charge-Charge Interactions 2. Hydrogen Bonds 3. Van der Waals Forces 4. Hydrophobic Interactions Hydrogen bonding is a common noncovalent interaction between biomolecules Figure 2.11 Hydrogen bonding between bases in DNA Figure 2.12 Van der Waals forces are weak noncovalent forces between atoms Figure 2.13 Amphipathic molecules, such as detergents, have both a polar and a nonpolar end. Figure 2.9 Detergents can form monolayers at the air-water interface Figure 2.10 Detergents can form micelles in aqueous solution Figure 2.10 Ionization of Water Water has a slight tendency to ionize Pages 41 and 42 Strong acids completely dissociate in water. Example: Hydrochloric acid (HCl) Weak acids dissociate in water with a characteristic acid dissociation constant (Ka). Example: Acetic acid, present in vinegar Relationship between pH and pKa Henderson – Hasselbalch equation Titration of acetic acid with aqueous base Figure 2.17 Titration of phosphoric acid, a polyprotic acid, with aqueous base Figure 2.19 1 2 3 4 5 6 7 8 9 10 1. Write the equilibrium reaction for the ionization of the weak acid. 2. What is the chemical structure of the conjugate base? 3. What is the pH of a solution containing equal amounts of the weak acid and the conjugate base? 4. What is the pH of a solution containing 10 times more weak acid than conjugate base? 5. What is the ratio of conjugate base to weak acid at pH = 7? 1. Write the equilibrium reaction for the ionization of the weak acid. 2. What is the chemical structure of the conjugate base? 1. Write the equilibrium reaction for the ionization of the weak acid. 2. What is the chemical structure of the conjugate base? 3. What is the pH of a solution containing equal amounts of the weak acid and the conjugate base? 4.8 CH3COOCH3COOH 4.8 1 4.8 0 1. Write the equilibrium reaction for the ionization of the weak acid. 2. What is the chemical structure of the conjugate base? 3. What is the pH of a solution containing equal amounts of the weak acid and the conjugate base? 4.8 CH3COOCH3COOH 4.8 1 4.8 0 4. What is the pH of a solution containing 10 times more weak acid than conjugate base? CH3COO- 4.8 CH3COOH 0.1 4.8 4.8 3.8 (-1) 5. What is the ratio of conjugate base to weak acid at pH = 7? 7 2.2 102.2 158 4.8 CH3COOCH3COOH CH3COOCH3COOH CH3COOCH3COOH CH3COOCH3COOH Titration of acetic acid with aqueous base Figure 2.17 Titration of acetic acid with aqueous base Figure 2.17 Buffering Region 1 pH unit from pKa Maintenance of Blood pH in Humans CO2 – Bicarbonate Buffer System Carbon dioxide – carbonic acid – bicarbonate buffer system maintains blood pH at 7.4 Figure 2.21 Regulation of blood pH in mammals Figure 2.22 Why is the CO2 – bicarbonate buffer system used in the human body? 1. The raw materials (CO2 and H2O) for the production of carbonic acid (H2CO3) are readily available. 2. The lungs and kidneys can easily adjust to ratio alterations between carbonic acid (H2CO3) and the conjugate base bicarbonate (HCO3-). Role of the lungs and kidneys in regulation of physiological pH Lungs Control the supply of H2CO3 in the blood by controlling the amount of CO2 exhaled. When the blood level of HCO3- decreases, the breathing rate is increased, increasing amount of CO2 expelled, decreasing H2CO3. If H2CO3 (CO2) increases it is called respiratory acidosis. If H2CO3 (CO2) decreases it is called respiratory alkalosis. Kidneys Control the concentration of HCO3-. If HCO3- is too high it is called metabolic alkalosis. If HCO3- is too low, it is called metabolic alkalosis. Blood Concentrations Ratio of HCO3- : H2CO3 = 10 : 1 This results in pH = 7.4 HCO3- = 24 - 27 mEq/L (mM) H2CO3 = 1.20 - 1.35 mEq/L (mM) Clinicians often monitor blood pH, HCO3- and CO2 concentrations. Non-graded Homework: Use Henderson-Hasselbalch equation to convince yourself this makes sense. Problem #11 at the end of the chapter.