WATER: Properties and Issues
advertisement

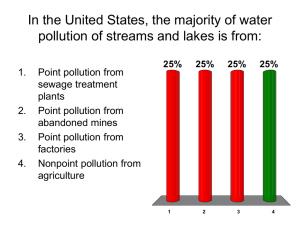
International Year of Chemistry (IYC) 2011 PowerPoint Pack Quarter One: Environment Water in Our World (W.O.W.) American Chemical Society, Department of Volunteer Support 1-800-227-5558 or e-mail us at oca@acs.org. Contact us for hands-on activities, contests, local contacts and additional information. American Chemical Society Wondering About Water Interesting facts about Water Over 70% of the Earth’s surface is covered with this liquid (oceans, rivers, lakes, etc.) The second most common form of water on Earth is ice (if all the ice melted – the sea-level would rise by 70 meters) Water is essential for life (most animals and plants contain more than 60% water by volume). Less than 1% of all water on Earth is available or clean enough for humans to drink. The rest is salty or frozen. Some of Water’s Physical Properties Boiling Point = 100 ⁰C (212 ⁰F) at 1 atmosphere of pressure Freezing Point = 0 ⁰C (32 ⁰F) at 1 atmosphere of pressure Density = 1 g/cc (at 4 ⁰C) Nearly Colorless Tasteless Odorless The Structure of Water – H2O Image from: http://www.chem1.com/acad/sci/aboutwater.html A Chemical Description of Water One atom of oxygen (O) is bound to two atoms of hydrogen (H) to form a 105 ⁰ angle “V” shape. Both H atoms are attached to one side of the O atom. This results in a molecule with a slight positive charge on one side of the molecule (H) and a slight negative charge on the other side (O) – this is called a dipole moment. Thus, water molecules tend to attract each other by a process called Hydrogen-Bonding - this gives water many unique properties. Hydrogen Bonding – creates attraction between water molecules Image from: http://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water.jpg Unique Properties of Water The only natural substance found as a liquid, solid, and gas at temperatures normally found on Earth Part of every living organism It dissolves nearly everything (universal solvent) It can absorb large amounts of heat – heat exchange between the atmosphere and oceans contribute greatly to Earth’s weather It’s molecules stick together to form beads or drops (high surface tension) – for example, rain drops Water Exists Primarily in Three States; Liquids, Solids, and Gases Image from: http://apollo.lsc.vsc.edu/.../chapter2/lat_heat3.html Another Unique and Important Property Most liquids contract when they get colder. Water contracts until it reaches 4 ⁰C . Then it expands until it is solid. The density of ice (0.915 g/cc) is less than that of liquid water (0.9999 g/cc) at 0 ⁰C. Its density at 4⁰C is 1.000 g/cc. This is why ice floats – if it didn’t, oceans, lakes, etc. would freeze from the bottom up and remain frozen – Earth would be a completely different planet - maybe uninhabitable by humans. American Chemical Society Water Matters Overview The amount of available water has not changed for thousands of years. Water is essential to the environment, human life, and industry. Approximately 1 billion people in over forty countries are currently under a water crisis. The increasing world population and the demand for better livelihoods globally, will continue to contribute to a worsening water crisis. Source: World Bank “Water and Development” Executive Summary, May 2010 Scarcity of Resource Each year millions of people die from diseases associated with inadequate water supply, sanitation and hygiene. Four of every ten people in the world do not have access to adequate sanitation. Two of every ten people have no source of safe drinking water. According to the United Nations World Water Development Report, by the year 2050, at least one in four people is likely to live in a country affected recurring shortages of freshwater. Source: http://www.un.org/waterforlifedecade/background.html Did You Know? Every day, 2 million tons of sewage and other types of pollution drain into the world's waters. Every year, more people die from unsafe water than from all forms of violence, including war. The most significant sources of water pollution are lack of effective management and poorly treated human, industrial, and agricultural wastes. Source: http://www.un.org/waterforlifedecade/quality.html Pollution Pollution refers to the introduction of harmful substances or products into the environment. Major water pollutants include microbes, nutrients, heavy metals, organic compounds, oil, sediments and heat (thermal pollution*). * Thermal pollution is typically the industrial release of heated water into a river, lake, or other body of water, causing a rise in temperature that endangers aquatic life Pollutants are usually the cause of extremely dreadful water quality conditions around the world.. Source: World Water Development Report 3 'Water in a Changing World' http://www.un.org/waterforlifedecade/quality.html Analyzing Water Depending on its use, water may have a variety of conditions for composition and purity. Types of analysis vary from simple field testing to determine a single property to laboratory based multi-property analysis. Some basic water quality measurements include pH, Acidity, Alkalinity, electrical conductivity, and water hardness. The pH of water measures its hydrogen ion concentration and indicates whether the sample is acidic, neutral or basic. Acidity of water measures its capacity to react with strong base to a designated pH. Alkalinity measures the acid-neutralizing capacity of water. It is attributed to the presence of hydroxide, carbonate, and bicarbonate ions. American Chemical Society Water: Getting It Clean, Keeping It Clean Potable Water and Its Importance Water is considered potable if it is safe for drinking and food preparation. http://en.wikipedia.org/wiki/File:Water_quality.jpg 18 Characteristics of Potable Water To be safe, drinking water must have minimal (safe) levels of the following: Disease causing microorganisms Cost to remove: low Dissolved chemicals Dissolved solids (salts that produce ions when dissolved) Toxic organic compounds Cost to remove: high to very high Suspended solids Cost for removal: low 19 Freshwater Sources Most public water sources are based on the low cost removal of suspended solids and microorganisms. Therefore, the water used in drinking water treatment plants must be fresh, meaning that it contains safe levels of dissolved chemicals. Sources of Freshwater Surface water: rivers, lakes, reservoirs, springs, etc. Ground water: wells 20 Public Drinking Water Treatment Prechlorination and aeration Prechlorination controls microbes such as algae that can clog a water treatment facility. Aeration converts iron and manganese to insoluble forms that can be removed. Coagulation (flocculation): conversion of small suspended particles (colloids) by causing them to aggregate into larger clusters. Usually encouraged by addition of a chemical flocculating agent such as alum (KAl(SO4)2.12H2O). Disinfection – remove microorganisms Chemical Methods: chlorine, ozone, chlorine dioxide Ultraviolet light, sunlight 21 Public Drinking Water Treatment (cont.) Sedimentation: allowing larger particulates and “floc” to settle out by gravity. Filtration: removal of additional particulates by passing water through filter. Sand is often used as a filtration medium A layer of activated carbon or anthracite coal can be added to remove dissolved organic materials. 22 Public Drinking Water Treatment Diagram http://www.americanchemistry.com/s_chlorine/docs/images/water_treatment.gif 23 Public Wastewater Treatment (Sewage Treatment) Pretreatment Debris Screening: large objects like wood, paper, toys, etc. are removed. Grit removal: large particles like sand and coffee grounds removed. Primary Treatment: Sedimentation: Fine particulates removed from tank bottom Oil and Grease can be skimmed off top 24 Public Wastewater Treatment (Sewage Treatment - Cont.) Secondary treatment Removes dissolved and suspended biological material Often utilizes trickle tanks that contain affixed microorganisms that digest biological material. Tertiary treatment: Disinfected Other 25 Public Wastewater Treatment (Sewage Treatment Figure) http://www.gic-edu.com/uploads/wastewatertreatment.jpg 26 Protecting Freshwater Sources It is important that everyone take responsibility for protecting our freshwater resources. Conserve water in our homes Avoid disposing of the following in sewer system Medications Grease and oil Pesticides Automotive and other chemicals Limit non-point source pollution: chemicals released in the environment at large that get washed into rivers, lakes, etc. by rainfall. 27 Summary Over 70% of the Earth’s surface is covered with this liquid (oceans, rivers, lakes, etc.) Water is essential for life (most animals and plants contain more than 60% water by volume). One atom of oxygen (O) is bound to two atoms of hydrogen (H) to form a 105 ⁰ angle “V” shape. Thus, water molecules tend to attract each other by a process called Hydrogen-Bonding - this gives water many unique properties. The amount of available water has not changed for thousands of years. Approximately 1 Billion people in over forty countries are currently under a water crisis. The most significant sources of water pollution are lack of effective management and poorly treated human, industrial, and agricultural wastes. Some water quality measurements include pH, Acidity, Alkalinity, electrical conductivity, and water hardness. Freshwater is a valuable resource that should be protected. Most freshwater must be treated before it is safe for drinking and food preparation. Methods for preparing potable water have to be adapted to each specific situation Methods such as desalination or atmospheric water harvesting are being developed and advanced to augment supplies of freshwater in the future.