Chapter 20 electrochemistry powerpoint
advertisement

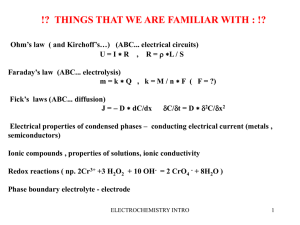
ELECTROCHEMISTRY INVOLVES TWO MAIN TYPES OF PROCESSES • A. Voltaic(galvanic) cells – which are spontaneous chemical reactions (battery) • B. Electrolytic cells – which are nonspontaneous and require external e− source (DC power source) • C. BOTH of these fit into the category entitled Electrochemical cells Electrochemistry Oxidation Numbers In order to keep track of what loses electrons and what gains them, we assign oxidation numbers. Electrochemistry Oxidation and Reduction • A species is oxidized when it loses electrons. Here, zinc loses two electrons to go from neutral zinc metal to the Zn2+ ion. Electrochemistry Oxidation and Reduction • A species is reduced when it gains electrons. Here, each of the H+ gains an electron and they combine to form H2. Electrochemistry Oxidation and Reduction • What is reduced is the oxidizing agent. H+ oxidizes Zn by taking electrons from it. • What is oxidized is the reducing agent. Zn reduces H+ by giving it electrons. Electrochemistry Assigning Oxidation Numbers 1. Elements in their elemental form have an oxidation number of 0. 2. The oxidation number of a monatomic ion is the same as its charge. Electrochemistry Voltaic Cells In spontaneous oxidation-reduction (redox) reactions, electrons are transferred and energy is released. Electrochemistry Voltaic Cells • We can use that energy to do work if we make the electrons flow through an external device. • We call such a setup a voltaic cell. Electrochemistry Voltaic Cells • A typical cell looks like this. • The oxidation occurs at the anode. • The reduction occurs at the cathode. Electrochemistry Voltaic Cells The flow of electrons is always from the anode to the cathode through the wire Electrochemistry Voltaic Cells Once even one electron flows from the anode to the cathode, the charges in each beaker would not be balanced and the flow of electrons would stop. Electrochemistry Voltaic Cells • Therefore, we use a salt bridge, usually a U-shaped tube that contains a salt solution, to keep the charges balanced. Cations move toward the cathode. Anions move toward the anode. Electrochemistry Voltaic Cells • In the cell, then, electrons leave the anode and flow through the wire to the cathode. • As the electrons leave the anode, the cations formed dissolve into the solution in the anode compartment. Electrochemistry Voltaic Cells • As the electrons reach the cathode, cations in the cathode are attracted to the now negative cathode. • The electrons are taken by the cation, and the neutral metal is deposited on the cathode. Electrochemistry Voltaic Cells Animation Electrochemistry Electromotive Force (emf) • Water only spontaneously flows one way in a waterfall. • Likewise, electrons only spontaneously flow one way in a redox reaction—from higher to lower potential energy. Electrochemistry Cell Potential (Electromotive Force (emf)) • The potential difference between the anode and cathode in a cell is called the electromotive force (emf). • It is also called the cell potential, and is designated Ecell. Electrochemistry Cell Potential (Electromotive Force (emf)) • Each potential is measured against a standard , which is the standard hydrogen electrode [consists of a piece • of inert Platinum that is bathed by hydrogen gas at 1 atm]. The hydrogen electrode is assigned a value • of ZERO volts. Electrochemistry Cell Potential (Electromotive Force (emf)) • standard conditions—1 atm for gases, 1.0M for solutions and 25°C for all (298 K) • • naught (°)--we use the naught to symbolize standard conditions [Experiencing a thermo flashback?] • That means • Ecell, Emf, or εcell become • Ecello , Emfo , or εcello when measurements are taken at standard conditions. You’ll soon learn how these change when the conditions are nonstandard! Electrochemistry Reduction Potential Chart • elements that have the most positive reduction potentials are easily reduced (in general, non-metals) • elements that have the least positive reduction potentials are easily oxidized (in general, metals) • The table can also be used to tell the strength of various oxidizing and reducing agents. • Can also be used as an activity series. Metals having less positive reduction potentials are more active and will replace metals with more positive potentials. Electrochemistry Standard Reduction Potentials Reduction potentials for many electrodes have been measured and tabulated. Electrochemistry Writing a Cell Diagram-Standard Cell notation “ion sandwich in alphabetical order” • The reduced species is written on the right hand side—this electrode is the cathode • The oxidized species is placed on the left hand side and this is the anode. • For example the cell diagram of a zinc and copper galvanic cell: • A vertical line (I ) represents the different phases present in each half cell-phase boundary. (if in same phase use comma instead) • A double vertical line II represents the salt bridge connecting the two half cells • Anode I solution II cathode solution I cathode Electrochemistry Zn(s) I Zn +2 II Cu+2 I Cu (s) • Zinc and Lead(s) (plumbous) • • Copper and iron+3 and +2 (use copper II) Electrochemistry What do you do when one of your electrodes lacks a SOLID METAL?? You will need an inert conductor (usually platinum ) put in parentheses (Pt) Different species of the same phase are separated by a comma • Fe+3 (aq) + Cu(s) Cu+2 + Fe2+ Electrochemistry Calculating Standard Cell Potential (Ecello , Emfo , or εcello ) • Decide which element is oxidized or reduced using the standard reduction potential chart. Element with more positive reduction potential gets reduced! • Write both equations as is from the chart with their voltage • Reverse the equation that will be oxidized and change the sign of the voltage • Balance the two half reactions BUT DO NOT MULTIPLY VOLTAGE VALUES • Add the two half reactions and their voltages together • Ecello = Eooxidation + Eoreduction where o means standard conditions 1atm , 1M and 25oC Electrochemistry Calculate Cell Potential E cello • Zinc and lead (s) Plumbous • Copper and iron+3 and +2 (use copper II) 1. H2 (g) + I2 (g) 2H+ (aq) + 2 I – (aq) Electrochemistry Calculate Cell Potential E cello Draw a diagram of the galvanic cell for the reaction and label completely • Fe+3 (aq) + Cu(s) Cu+2 + Fe2+ Electrochemistry Cell Potential , Electrical Work, And Free energy Cell potential is measured in volts (V). It is the work that can be accomplished when electrons are transferred through a wire. Work(J) 1V=1 Charge ( C) Electrochemistry Cell Potential , Electrical Work, And Free energy 1 joule of work is required or produced when one coloumb of charge is transferred between two points Work(J) 1V=1 Charge ( C) Electrochemistry Cell Potential , Electrical Work, And Free energy • Using the table of standard reduction potentials, calculate DG o for the reaction: • Cu+2(aq) + Fe(S) Cu (s) + Fe+2 (aq) • • IS it spontaneous? Electrochemistry Cell Potential , Electrical Work, And Free energy • • • • • • DG o = - nFEo G= Gibb’s Free energy n= numbers of moles of electrons F= Faraday constant 96500 J/V . Mol The redox reaction will be spontaneous when DG o = _________ The redox reaction will be spontaneous when Eo = __________ Electrochemistry Cell Potential , Electrical Work, And Free energy DG o = - nFEo + DG o = - RT ln K = • You can derive the equation: for Ecell at standard conditions at equilibrium Eo = RT ln K nF Electrochemistry o E K Greater than 1 =1 Less than one = RT ln K nF Eo Positive DG o negative 0 Negative 0 positive Conclusion Electrochemistry •Nernst Equation E = E − RT nF ln Q •Why? It is used to calculate the voltage generated by the combination of two half-cells when the conditions are not standard!!!!!!! • n= numbers of electrons transferred between species • F= Faraday constant 96500 J/V . mol • R=ideal gas constant 8.314 /K mol • T= Kelvin Temperature • Eo= the voltage generated if the conditions » were standard • Q= reactions quotient [products]x • [reactants]y Electrochemistry If the reaction below is carried out using solutions that are 5.0 M Zn +2 and 0.3 M Cu+2 at 298 K, what is the actual cell voltage? Cu(s) + Zn+2 Zn (s) + Cu+2 • Step 1: Work out Eo cell assuming standard conditions: • • • Step 2: Calculate Q (don’t forget _____ and _______ are omitted from Q) • • • • Step 3: Find n • • Step 4: Plug everything in! Electrochemistry (Answer 1.06 V) Assigning Oxidation Numbers 3. Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. Oxygen has an oxidation number of −2, except in the peroxide ion in which it has an oxidation number of −1. Hydrogen is −1 when bonded to a metal, +1 when bonded to a nonmetal. Electrochemistry Assigning Oxidation Numbers 3. Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. Fluorine always has an oxidation number of −1. The other halogens have an oxidation number of −1 when they are negative; they can have positive oxidation numbers, however, most notably in oxyanions. Electrochemistry Assigning Oxidation Numbers 4. The sum of the oxidation numbers in a neutral compound is 0. 5. The sum of the oxidation numbers in a polyatomic ion is the charge on the ion. Electrochemistry Balancing Oxidation-Reduction Equations Perhaps the easiest way to balance the equation of an oxidation-reduction reaction is via the half-reaction method. Electrochemistry Balancing Oxidation-Reduction Equations This involves treating (on paper only) the oxidation and reduction as two separate processes, balancing these half reactions, and then combining them to attain the balanced equation for the overall reaction. Electrochemistry Half-Reaction Method 1. Assign oxidation numbers to determine what is oxidized and what is reduced. 2. Write the oxidation and reduction halfreactions. Electrochemistry Half-Reaction Method 3. Balance each half-reaction. a. b. c. d. Balance elements other than H and O. Balance O by adding H2O. Balance H by adding H+. Balance charge by adding electrons. 4. Multiply the half-reactions by integers so that the electrons gained and lost are the same. Electrochemistry Half-Reaction Method 5. Add the half-reactions, subtracting things that appear on both sides. 6. Make sure the equation is balanced according to mass. 7. Make sure the equation is balanced according to charge. Electrochemistry Half-Reaction Method Consider the reaction between MnO4− and C2O42− : MnO4−(aq) + C2O42−(aq) Mn2+(aq) + CO2(aq) Electrochemistry Half-Reaction Method First, we assign oxidation numbers. +7 +3 +2 +4 MnO4− + C2O42- Mn2+ + CO2 Since the manganese goes from +7 to +2, it is reduced. Since the carbon goes from +3 to +4, it is oxidized. Electrochemistry Oxidation Half-Reaction C2O42− CO2 To balance the carbon, we add a coefficient of 2: C2O42− 2 CO2 Electrochemistry Oxidation Half-Reaction C2O42− 2 CO2 The oxygen is now balanced as well. To balance the charge, we must add 2 electrons to the right side. C2O42− 2 CO2 + 2 e− Electrochemistry Reduction Half-Reaction MnO4− Mn2+ The manganese is balanced; to balance the oxygen, we must add 4 waters to the right side. MnO4− Mn2+ + 4 H2O Electrochemistry Reduction Half-Reaction MnO4− Mn2+ + 4 H2O To balance the hydrogen, we add 8 H+ to the left side. 8 H+ + MnO4− Mn2+ + 4 H2O Electrochemistry Reduction Half-Reaction 8 H+ + MnO4− Mn2+ + 4 H2O To balance the charge, we add 5 e− to the left side. 5 e− + 8 H+ + MnO4− Mn2+ + 4 H2O Electrochemistry Combining the Half-Reactions Now we evaluate the two half-reactions together: C2O42− 2 CO2 + 2 e− 5 e− + 8 H+ + MnO4− Mn2+ + 4 H2O To attain the same number of electrons on each side, we will multiply the first reaction by 5 and the second by 2. Electrochemistry Combining the Half-Reactions 5 C2O42− 10 CO2 + 10 e− 10 e− + 16 H+ + 2 MnO4− 2 Mn2+ + 8 H2O When we add these together, we get: 10 e− + 16 H+ + 2 MnO4− + 5 C2O42− 2 Mn2+ + 8 H2O + 10 CO2 +10 e− Electrochemistry Combining the Half-Reactions 10 e− + 16 H+ + 2 MnO4− + 5 C2O42− 2 Mn2+ + 8 H2O + 10 CO2 +10 e− The only thing that appears on both sides are the electrons. Subtracting them, we are left with: 16 H+ + 2 MnO4− + 5 C2O42− 2 Mn2+ + 8 H2O + 10 CO2 Electrochemistry Balancing in Basic Solution • If a reaction occurs in basic solution, one can balance it as if it occurred in acid. • Once the equation is balanced, add OH− to each side to “neutralize” the H+ in the equation and create water in its place. • If this produces water on both sides, you might have to subtract water from each side. Electrochemistry Standard Hydrogen Electrode • Their values are referenced to a standard hydrogen electrode (SHE). • By definition, the reduction potential for hydrogen is 0 V: 2 H+ (aq, 1M) + 2 e− H2 (g, 1 atm) Electrochemistry Standard Cell Potentials The cell potential at standard conditions can be found through this equation: Ecell (cathode) − Ered (anode) = Ered Because cell potential is based on the potential energy per unit of charge, it is an intensive property. Electrochemistry Cell Potentials • For the oxidation in this cell, Ered = −0.76 V • For the reduction, Ered = +0.34 V Electrochemistry Cell Potentials Ecell = Ered (cathode) − Ered (anode) = +0.34 V − (−0.76 V) = +1.10 V Electrochemistry Oxidizing and Reducing Agents • The strongest oxidizers have the most positive reduction potentials. • The strongest reducers have the most negative reduction potentials. Electrochemistry Oxidizing and Reducing Agents The greater the difference between the two, the greater the voltage of the cell. Electrochemistry Nernst Equation • Remember that DG = DG + RT ln Q • This means −nFE = −nFE + RT ln Q Electrochemistry Nernst Equation Dividing both sides by −nF, we get the Nernst equation: RT ln Q E = E − nF or, using base-10 logarithms, 2.303 RT ln Q E = E − nF Electrochemistry Concentration Cells • Notice that the Nernst equation implies that a cell could be created that has the same substance at both electrodes. would be 0, but Q would not. • For such a cell, Ecell • Therefore, as long as the concentrations are different, E will not be 0. Electrochemistry Applications of Oxidation-Reduction Reactions Electrochemistry Batteries Electrochemistry Alkaline Batteries Electrochemistry Hydrogen Fuel Cells Electrochemistry Corrosion and… Electrochemistry …Corrosion Prevention Electrochemistry