Chapter 20 Electrochemistry
advertisement

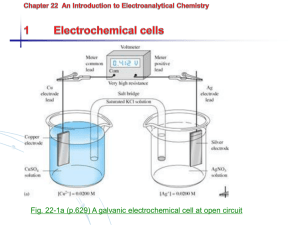
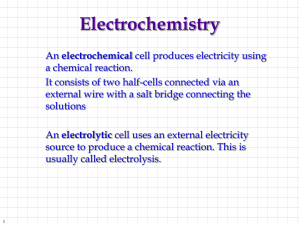
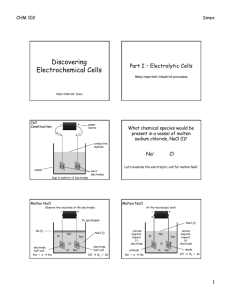
Chapter 20 Electrochemistry 20.1 Introduction to Electrochemistry Electrochemistry • The branch of chemistry that deals with electricity-related applications of oxidationreduction reactions. • Electrochemical Cells: A system of electrodes and electrolytes in which either chemical reactions produce energy or an electrical current produces chemical change Components of Electrochemical Cells Cu Electrode Cathodewhere reduction takes place Conducting Wire Electrolyte Sol’n ZnSO4 Electrode: conductor used to establish electrical contact with a nonmetallic part of the circuit. Electrolyte Sol’n CuSO4 Zn Electrode Anode- where oxidation takes place Half-Cell: a single electrode immersed in a solution of its ions Cu Electrode Cathode- written as Cu+2/Cu Overall Cell Written as: anode | cathode Zn | Cu Zn Electrode Anode- written as Zn+2/Zn Half-Cell: a single electrode immersed in a solution of its ions Chapter 20 Electrochemistry 20.2 Voltaic Cells Electrochemistry Porous barrier which prevents the spontaneous mixing of the aqueous solutions in each compartment, but allows the movement of ions in both directions to maintain electrical neutrality Voltaic / Galvanic Cell • A chemical rxn that results in a voltage due to a transfer of electrons Rxns that produce voltage spontaneously Batteries Zn → Zn+2 + 2e- 2MnO2 + H2O + 2e- → Mn2O3 + 2OH - • Two or more dry voltaic cells • Zinc-Carbon Battery Batteries Zn + 2OH → Zn(OH)2 + - 2e- 2MnO2 + H2O + 2e- → Mn2O3 + 2OH- • Alkaline Battery- no carbon rod, smaller Batteries• Mercury Battery- no Zn + 2OH - → Zn(OH)2 + 2e- HgO + H2O + 2e- → Hg + 2OH - carbon rod, smallest Fuel Cells Cathode: O2 + 2H2O + 4e- → 4OH – Anode: 2H2 + 4OH – → 4e- + 4H2O Net: 2H2 + O2 → 2H2O • A voltaic cell where reactants are constantly supplied and products are removed. Rxns that turn chemical energy into electrical energy 12 Corrosion Formation of Rust: 4Fe (s) + 3O2 (g) + xH2O → 2Fe2O3∙xH2O Anode: Fe (s) → Fe+2 (aq) + 2eCathode: O2 (g) + 2H2O (l) + 4e- → 4OH – Prevention of Corrosion Galvanizing Process by which iron or any metal is coated with zinc. Cathodic Protection Since zinc is more easily oxidized, it is a sacrificial anode. Electrode Potentials • Reduction Potential: the tendency for the halfreaction to occur as a reduction half-reaction in an electrochemical cell. • Electrode Potential: the difference in potential between an electrode and its solution • Potential Difference (Voltage): a measure of the energy required to move a certain electric charge between the electrodes, measured in volts. • Standard Electrode Potential (E°): a half-cell measured relative to a potential of zero for the standard hydrogen electrode (SHE) Standard Electrode Potential, E° • Positive E° means hydrogen is more willing to give up its electron, so positive reduction potentials are favored. Naturally occurring rxns have a positive value. E° cell = E° cathode - E° anode • Negative E° means the metal electrode is more willing to give up its electron, this is not favored. These rxns prefer oxidation over reduction. Standard Electrode Potential, E° • When a half-cell is multiplied by a constant (for balancing) the E° value is NOT multiplied! • When a rxn is reversed (flipped) the sign of the E° value switches. • In a voltaic cell, the half-rxn with the more negative standard electrode potential is the anode, where oxidation occurs. Cell Potential • The potential voltage a rxn can produce. Cu2+ + 2e- Cu Eo = .34 V Ag+ + e- Ag Eo = .80V Because this is a spontaneous process: (Ag+ + e- Ag) x 2 Eo = .80V Cu Cu2+ + 2e- Eo = -.34 V Cu + 2Ag+ Cu2+ + 2Ag Eo = .46 V Reduction potentials Since both rxns are reduction, one must be oxidation, flip it, positive voltage must result from spontaneous rxns Cell Potential • The potential voltage a rxn can produce. Na+ + e- Na Eo = -2.71 V Cl2 + 2e- 2Cl- Eo = 1.36 V Because this is nonspontaneous process: (Na+ + e- Na) x 2 Eo = -2.71 V 2Cl- Cl2 + 2e- Eo = -1.36 V 2Na+ + 2Cl- 2Na + Cl2 Eo = -4.07 V Nonspontaneous, must end in negative voltage. Flip one to become oxidation. ** Fuel Cell! Chapter 20 Electrochemistry 20.3 Electrolytic Cells Electrochemistry • When electric voltage is used to produce a redox reaction, it is called electrolysis Electrolytic Cell Rxns that require an energy source to react Batteries Discharge Cycle Rxn: Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O • Car Batteryrechargeable b/c the alternator reverses the ½ rxns and regenerates the reactants. Electroplating • An electrolytic process in which a metal ion is reduced and a solid metal is deposited on a surface • Typically, an inactive metal is able to be ionized and then deposited on the surface of a more active metal to prevent corrosion. Anode Silver ions are reduced at the cathode: Ag+ + 1e- → Ag Silver atoms are oxidized at the anode: Ag → Ag + + 1e- Voltaic vs. Electrolytic • If the positive battery terminal is attached to the cathode of a voltaic cell, and the negative terminal is attached to the anode, the flow of electrons will change directions. • Electrolytic cells need the electrodes attached to a battery, where voltaic is its own source of electrical power. Voltaic = spontaneous chemical energy → electrical energy Electrolytic = non-spontaneous electrical energy → chemical energy Electrolysis Anode: 6H2O → O2 + 4e- + 4H3O+ Cathode: 4H2O + 4e- → 2H2 + 4OH – Using a current to generate a redox reaction which otherwise would have a negative cell potential. i.e. electroplating & rechargeable batteries.