Introduction to electrochemistry lab
advertisement

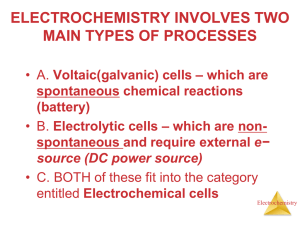
!? THINGS THAT WE ARE FAMILIAR WITH : !? Ohm’s law ( and Kirchoff’s…) (ABC... electrical circuits) U = I R , R = L / S Faraday’s law (ABC... electrolysis) m = k Q , k = M / n F ( F = ?) Fick’s laws (ABC... diffusion) J = D dC/dx C/t = D 2C/x2 Electrical properties of condensed phases – conducting electrical current (metals , semiconductors) Ionic compounds , properties of solutions, ionic conductivity Redox reactions ( np. 2Cr3+ +3 H2O2 + 10 OH- = 2 CrO4 - + 8H2O ) Phase boundary electrolyte - electrode ELECTROCHEMISTRY INTRO 1 Me+ transport Me Me+ electrode electrolyte Charge transfer Oxidation – reduction reaction rate Diffusion transport rate ELECTROCHEMISTRY INTRO 2 Each stage can determine the overall reaction rate 1. „obligatory” stages charge transfer Transport (diffusion, convection , migration ) 2. other possible stages Chemical reaction before or after (c t) Crystallisation of new phases Adsorption at the electrode ELECTROCHEMISTRY INTRO 3 = A ne B Reaction rate v = ∆ cA / ∆t , ( or v = kA-B · cA ) ( cA - volumetric concentration – we must do something about it) In electrode kinetics the transferred charge is a measure of reaction rate Following Faraday’s law: mA = kF · I · ∆t = k · Q (here k – electrochem equivalent, not reaction rate constant) And back to general reaction rate formula mA = cA · V or cA surface · S = kF · I · ∆t v= k·I·/S v ( mol · s-1 m) = kF (mol/C) · j , j – current density (A/m2) ELECTROCHEMISTRY INTRO 4 CURRENT DENSITY = MEASURE OF ELECTRODE PROCESS RATE And what makes the reaction happen at all?? Equilibrium – no products ( is anything happening?) Deviation from equilibrium - energy impuls needed Reaction – transformation to new equilibrium state What might be an energy impuls? ELECTROCHEMISTRY INTRO 5 energy state of a particle – chemical potential μi= μ o + RT ( ai) Charged particle - electrochemical potential , possible responce to electrical field φ= φo + RT/nF ln ( ai(n+) ) Equilibrium - equal potentials of a particle in two phases (electrode – electrolyte) E = E0 + RT/nF ln ( aelectrode / aelectrolyte ) Change in concentration, temperature ENERGY IMPULS Overpotential applied to the electrode ELECTROCHEMISTRY INTRO 6 At equilibrium Redox transitions on molecular scale Identical overall charge for oxidation and reduction jk = ja , overall current density jk - ja = 0 At overpotential ∆E j = jk - ja ≠ 0 as measure for reaction rate, so j/nF = v = krr × C ELECTROCHEMISTRY INTRO 7 Reaction rate constant - overpotential: krr = ks exp[ α n F ΔE / RT] (one equilibrium – two constants: anodic and cathodic) Combining v = .. And k = …. (To get current-overpotential dependence) i = nF S ks [ cutl exp(-αn F ΔE / RT ) – cred (βn F ΔE / RT)] where ks - standard reaction rate constant α i β coefficients for energy barrier symmetry ΔE overpotential ELECTROCHEMISTRY INTRO 8 • Electrode process – heterogenous, charge transfer at phase boundary + transport • Electrode = element of electrical circuit • Measurement = two electrodes form a cell • Circuit – measurable : voltage and current • Difference in V/I response for a.c and d.c. ELECTROCHEMISTRY INTRO 9 Transport properties • Structure of electrolytes, dissociation • • Movement of ionic species • Mobility, velocity of part i vi = E · ui • Conductivity = e · Ni · zi · ui • Transference number ELECTROCHEMISTRY INTRO 10 Cell voltage or electrode potential • Equilibrium at the electrode – Nernst pot. • Overpotential – driving force for the reaction • Current – electrical measure of reaction rate • Voltage – measure of potential difference ! • For kinetics – we must know the potential of a single electrode! ELECTROCHEMISTRY INTRO 11 3 –electrode cell •3-electrode cells : WE and CE - „working circuit” reactions at electrodes , current flow WE- RE - measuring circuit , high input impedance on RE no current flow •Reference electrodes : very precise potential, examples : Hg/ Hg2Cl2 , Ag/ AgCl , •Quasi-reference : W, Ta, other non-reactive metals idea : stable potential, easy assembly, •Function current-potential – diffusion and kinetics in the cell must be described electroanalysis ELECTROCHEMISTRY INTRO 12 Electrolytic cell and potentiostat Electrodes: Meas. Parameters: CE - counter RE - reference WE - working E - WE potential Ez - applied potential I - current in CE-WE circuit I RE CE E WE Cell Ez Potentiostat ELECTROCHEMISTRY INTRO 13 EIS • Electrotechnical aspect : a.c.circuit • Electrochemical aspect : approximation of electrode process with circuit elements Charge transfer Conductivity Resistance of layer Resistances R Z=R ELECTROCHEMISTRY INTRO 14 Double layer capacity Capacity of layers Capacities C Z = -j/C Diffusion phenomena Roughness of surface Inhomogenity of layer Constant phase element Admittance Y = Yo (j)n for n=0 resistance For n=1 capacity Corrosion processes (many reactions and equilibria) Inductance L Z = j L ELECTROCHEMISTRY INTRO 15 Equivalent circuits • Electrical model of electrode • Connections in series and parallel – interpretation of consecutive or simultaneous reactions / phenomena • Physical sense vs. numerical possibilities ELECTROCHEMISTRY INTRO 16 Our lab sessions EIS – dr Regina Borkowska ( 5h basic electrode kinetics) Voltammetry – dr Regina Borkowska 5h Conducting polymers dr M. Siekierski 5h Batteries – dr Marek Marcinek (5h basic cells + 5h Li- cells) Transference numbers – Msc Michał Piszcz(5h diffusion coefficient + 5h transference numbers in Li systems) Ion associations – Dr Leszek Niedzicki (5h Fuoss-Kraus formalism – electrochemical approach) Corrosion dr Andrzej Królikowski 5h Instructions and auxillary materials: download from http://pirg.ch.pw.edu.pl/ ELECTROCHEMISTRY INTRO 17