CELLULAR TRANSPORT
Diffusion and Osmosis
Our goal today: To
describe how
OSMOSIS happens.
Osmosis is the diffusion of
water across a membrane.
Key Concepts
• To understand osmosis, you must
first understand the following:
– Diffusion
– Properties of a cell membrane
– Effects of solute concentration on
water molecules
Source Citation: Text excerpts from Miller and Levine, 2006, Biology, Pearson, Prentice Hall.
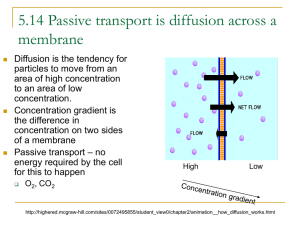
DIFFUSION
Particles move constantly. They bump into each
other and spread out randomly. As a result, the
particles tend to move from an area where they
are more concentrated, to an area where they are
less concentrated. This is called DIFFUSION.
Drop of dye
Pure water
Dye evenly
dispersed in
water
THE CELL’S ENVIRONMENT
The cytoplasm of a cell contains a solution
of many different substances in water.
Recall that a solution is a mixture of two or
more substances. The CONCENTRATION
of a solution is the mass of the solute in a
given volume of solution.
Same volume
Less
concentrated
More
concentrated
EQUILIBRIUM
Drop of dye
Dye evenly
dispersed in
water
Pure water
When the concentration of the solute is the
same throughout a system, the system has
reached EQUILIBRIUM. Keep in mind, however,
that even in this state, the molecules are still in
motion.
Back to “Key Concepts”
THE CELL MEMBRANE
One of the most important functions of
the cell membrane is to regulate the
movement of dissolved molecules from
the liquid on one side of the membrane to
the liquid on the other side.
Source Citation: "Kimball, John W.
'Transport Across Cell Membranes.'
Kimball's Biology Pages. August 17, 2002.
http://users.rcn.com/jkimball.ma.ultranet/Bi
ologyPages/D/Diffusion.html (January
2003)." Science Resource Center.
Thomson Gale. 15 April 2007
Because cells are surrounded by liquid, and have
liquid inside of them, diffusion of molecules
dissolved in the liquid is constantly occurring
across the cell membrane. The direction in
which they move depends on the concentration
gradient. Not all molecules can diffuse across
the membrane; some are too large, some are too
strongly charged.
But molecules such as water, oxygen, and
carbon dioxide (which are small and not
strongly charged) can easily pass across
the bilayer. For this reason, cell membranes
are called SELECTIVELY PERMEABLE.
Lipid-soluble
molecules,
O2, CO2, H2O
(outside)
(inside)
Back to “Key Concepts”
OSMOSIS
Click here to
return to
objective.
Image from Miller and Levine, 2006, Biology, by Pearson Prentice Hall
When water diffuses across the membrane,
it is called OSMOSIS. Water will move
across a membrane until equilibrium is
reached. Click here to see osmosis in
action (X-out when you are done).
THE CONCENTRATION OF
WATER
The concentration of water can change by
adding solutes to a fixed volume of liquid.
For example, if we keep
the volume of a solution
CONSTANT, and
increase the amount of
sugar or salt, then we
decrease the
concentration of water.
Less
solute,
more
water
Volume
does not
change
More
solute,
less water
THE CONCENTRATION OF
WATER
Likewise, if we keep the volume of a
solution constant, and decrease the
amount of sugar or salt, then we increase
the concentration of water.
Volume
does not
change
More solute,
less water
Less solute,
more water
Image from Purves et al., Life: The Science of Biology, 4th Edition, by
Sinauer Associates
Click here to
learn key terms.
If cells are placed in a
solution that is low in
solutes (or high in
water), it causes the
cell to swell. If cells
are placed in a
solution that is high
in solutes (or low in
water), it causes the
cell to shrink.
X-out when you are done
Back to “Key Concepts”
Demonstration
Watch what happens when a plant cell is
placed into a solution of salt water. Click
the button below.
Elodea leaf in salt water
X-out when you are done
Did you miss something?
• Check one or all of the interactive links
below to help you fill in any “blanks” you
might have:
– All about Cell Transport
– Diffusion and Osmosis (stop at “Calculating Water Potential)
– Osmosis
– Key terms
X-out of each activity to return to this Power Point
APPLICATION
How can we apply what we now know about
diffusion to our bodies?
An important part of
respiration is the uptake of
oxygen and release of
carbon dioxide by our cells.
These gases are exchanged
in small structures in our
lungs called alveoli.
Click here to see
an animation of a
human lung
Click here to see a
close-up view of
gas exchange
APPLICATION
Where else does diffusion and osmosis
happen?
Our kidneys have the important
job of removing wastes such as
urea from our bodies. Urea is
excreted in urine. Urine is a
product of diffusion and
osmosis.
Click here to get
a closer look at
kidney function
Clickhttp://www.kidneypa
tientguide.org.uk/HDanim
.php here to see how
dialysis works in patients
HSA Review
Which of these is the process by which
water moves across a selectively
permeable membrane?
A
B
C
D
osmosis
transpiration
capillary action
active transport
You are looking for the process that
describes the movement of water
down its concentration gradient. It
does not require the cell to use any
energy.
Osmosis is the diffusion of water
across a cell membrane.
HSA Review
The exchange of oxygen and carbon dioxide
between the body and the air occurs in the
lungs. This exchange of gases takes place at
the cellular level. What part of the cell is
primarily responsible for this exchange?
F
G
H
J
the cell membrane
the nucleus
the cell wall
the ribosome
You are looking for the part of the cell
that is in direct contact with the cell’s
environment and is permeable to
various molecules.
The cell membrane is like the
gatekeeper of the cell; its structure
allows certain molecules to move into
and out of the cell.