Molecular Luminescence
Spectroscopy
Lecture Date: February 4th, 2013
Luminescent Electronic Processes
Luminescence:
radiation produced by a chemical reaction
or internal electronic process, possibly following
absorption. Includes fluorescence, phosphorescence, and
chemiluminescence.
Fluorescence:
absorption of radiation to an excited state,
followed by emission of radiation to a lower state of the
same multiplicity
– Occurs about 10-5 to 10-8 seconds after photon absorption
Phosphorescence:
absorption of radiation to an excited
state, followed by emission of radiation to a lower state of
different multiplicity
– Occurs about 10 to 10-5 seconds after photon absorption
History of Fluorescence Spectroscopy
1845: W. Herschel first
observes blue fluorescence
from a quinine solution excited
by sunlight
1852: Stokes first explains
fluorescence in quinine as
arising from frequency
differences in light
A. Jablonski
1900’s: Jablonski develops theory of
excited state processes and anisotropy
1950’s: first fluorescence spectrometers
developed at NIH
J. F. W. Herschel
G. G. Stokes
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3 rd Ed., Springer, 2006, pg. 2-7.
Molecular Fluorescence
Non-resonant fluorescence is a phenomenon in which
absorption of light of a given wavelength by a fluorescent
molecule is followed by the emission of light at longer
wavelengths (applies to molecules)
Why use fluorescence?
One key reason is that it is not a
difference method!
Method
Mass detection
limit (moles)
Concentration
detection limit
(M)
Advantage
UV-Vis
10-13 to 10-16
10-5 to 10-8
Universal
fluorescence
10-15 to 10-17
10-7 to 10-9
Sensitive
Singlet and Triplet States (Two Electron Systems)
Electrons are spin ½ particles
Singlet state: spins are
paired, no net angular
momentum (and no net
magnetic field), one
eigenstate ( |0,0 )
Triplet state: spins are
unpaired, net angular
momentum (and net magnetic
field), three eigenstates (|1,-1
, |1,0, |1,1 )
Theory of Molecular Fluorescence
A typical Jablonski energy diagram:
Notation: S2, S1 = singlet states, T1 = triplet state
Fluorescence is a singlet-to-singlet state process; phosphorescence
converts the singlet to a triplet state via intersystem crossing
Excitation directly to a triplet state is forbidden by selection rules.
Molecular Fluorescence Terminology
Quantum yield (): the ratio of molecules that luminescence to the
total # of molecules
Resonance fluorescence: fluorescence in which the emitted radiation
has the same wavelength as the excitation radiation
Internal conversion: after absorption of the photon, molecules in
condensed phases often relax to the lowest vibrational level of S1
within 10-12 s
Intersystem crossing: a transition in which the spin of the electron is
reversed (change in multiplicity in molecule occurs, singlet to triplet).
– Enhanced if vibrational levels overlap or if molecule contains
heavy atoms (halogens), or if paramagnetic species (O2) are
present.
Dissociation: excitation to vibrational state with sufficient energy to
break a chemical bond
Pre-dissociation: relaxation to vibrational state with sufficient energy
to break a chemical bond
The Stokes Shift and Mirror Image Rule
Stokes shift:
a shift to
longer wavelengths
between excitation and
emitted radiation
Mirror image rule:
the FL
emission spectrum is the
mirror image of the
absorption spectrum.
The rule is often violated!
The mirror image rule is
a consequence of the
Frank-Condon principle
Basic Fluorescence Spectrometers
Typical layout of major components
90° angle or
front facing geometry
Sample
Radiation
Source
and
Monochromator 1
Wavelength
Selector
Monochromator 2
Detector
(Photomultiplier
tube)
Predicting the Fluorescence of Molecules
Some things that improve fluorescence:
– Low energy * transitions
– Rigid molecules (e.g. biphenyl and fluorene)
– Transitions that don’t have competition. Example: fluorescence
sometimes does not occur after absorption of UV wavelengths <
250 nm because the radiation has too much energy (>100
kcal/mol). Dissociation occurs instead (but multiphoton
excitation may be possible).
– Chelation to metals
biphenyl
fluorescence QE = 0.2
fluorene
fluorescence QE = 1.0
Intersystem crossings reduce fluorescence (competing
process is phosphorescence).
Predicting the Fluorescence of Molecules
More factors that affect fluorescence:
– decrease temperature = increase fluorescence
– increase viscosity = increase fluorescence
– pH dependence for acid/base compounds (titrations)
Calculation of fluorescence using DFT
– Possible using modified TDDFT approaches – must include both
vibrational and electronic calculations
R. Improta et al., J. Phys. Chem. B, 2007, 111, 14080-14082.
Fluorophores
Two major classes:
– Intrinsic: the fluorescence occurs naturally in the molecule. The
indole group in tryptophan (Trp) residues in proteins absorbs at
280 nm and emits at 340 nm
– Extrinsic: the fluorophore is added to a sample. For example, 1anilinonaphthalene-6-sulfonic acid (ANS) and 2-(para-toluidinyl)
naphthalene-6-sulfonic acid (TNS) fluorophores used to noncovalently label proteins.
Types of extrinsic fluorescent species:
–
–
–
–
Conjugated organic molecules
Lanthanide complexes
Quantum dots
Nanotubes
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3rd Ed., Springer, 2006, pg. 15.
Organic Small Molecule Fluorophores
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3rd Ed., Springer, 2006, pg. 2.
Quantum Dots as Fluorophores
Quantum dots and other
nanoparticle semiconductors are a
recent addition (~1998) to the world
of fluorophores
CdSe and other semiconductors
exhibit strong, narrow FL emission
with maxima controlled by particle
size
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3rd Ed., Springer, 2006, pg. 675-678.
Nanotubes as Fluorophores
Carbon and boron nitride singlewalled nanotubes (SWNTs) are
currently being explored as red to
near infrared fluorophores
Carbon SWNT
n=10, m=10
Length = 49.19 Å
SWNTs can be functionalized with
groups capable of molecular
recognition
Single-molecule emission spectra
for carbon SWNTs (n, m):
L. J. Carlson and T. J. Krauss, Photophysics of Individual Single-Walled Carbon Nanotubes, Acc. Chem. Res., 2008, 41, 235-243, http://dx.doi.org/10.1021/ar700136v
Quenching of Fluorescence
Quenching:
a process that reduces fluorescence intensity
– Collisional: excited state of the molecule is deactivated by
collision with another molecule in solution (explained by the
Stern-Vollmer equation)
– Static: excited state intensity reduced by formation of a complex
Most common (unintentional) quencher – dissolved
oxygen (O2)
Quenching of fluorophores is commonly used to probe
“accessibility,” e.g. by adding a quencher in varying
amounts and observing its effects on a protein fluorophore
to determine its location
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3 rd Ed., Springer, 2006, pg. 15, pp. 278-286.
Time-Resolved Fluorescence Spectroscopy
Up to this point, we’ve been discussing steady-state
fluorescence spectroscopy.
Time-resolved fluorescence spectroscopy: the study of
fluorescence spectra as a function of time (usually ps to
ns), to measure fluorescence lifetimes ()
Demands a different experimental approach than steadystate fluorescence measurements
Two major approaches:
– Time domain: sample is excited with a short pulse of light, and
the decay in FL is observed. The most common approach is
time-correlated single-photon counting (TCSPC).
– Frequency domain: sample is excited with amplitude modulated
light (typically with a frequency of 100 MHz), causing the
emission to respond at the same frequency but delayed by the
lifetime of the fluorophore (leading to a phase shift that is
measured to get to the lifetime).
Fluorescence Lifetime Measurements
Different species have different lifetimes.
Here the Trp
residues in a protein, in the presence of a collisional
quencher, shows a biexponential decay:
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3 rd Ed., Springer, 2006, pg. 101.
Fluorescence Lifetime Measurements
The latest detectors allow for full
emission spectra at each time
point, which in turns allows for
observation of excited state
complex formation.
Here a dye is observed to form a
charge-transfer (CT) exciplex and
then engages in solvent-induced
relaxation
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3 rd Ed., Springer, 2006, pg. 126.
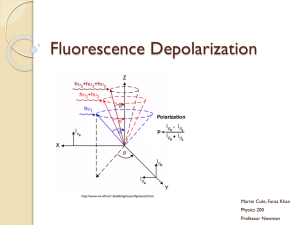
Fluorescence Anisotropy
Fluorophores prefer to absorb photons with a electric field vector
aligned to the electric transition moment of the fluorophore (which
is oriented relative to the molecule).
Selective excitation of a subset of fluorophores can be achieved
with polarized light, allowing the loss of polarization to be studied.
Time-resolved fluorescence anisotropy is used to study proteinprotein interactions and mobility of membrane proteins.
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3rd Ed., Springer, 2006, pg. 12-16..
Resonance Energy Transfer (RET)
The RET (or Fluorescence Resonance Energy Transfer,
FRET) method is possible when the emission spectrum of
a fluorescent donor and the absorption spectrum of an
acceptor (not necessarily fluorescent) overlap.
The RET effect is predicted to have a rate (kT) related to
the distance (r) between the donor and acceptor groups:
Förster distance
1 R0
kT r
D r
6
The FL lifetime of the donor in the
absence of RET
J. La kowicz, “Principles of Fluorescence Spectroscopy”, 3rd Ed., Springer, 2006, pg. 15.
Multiphoton-Excited Fluorescence
Known as MPE (as opposed to the
usual 1PE)
Lots of energy required, achieved
via femtosecond-pulse lasers
Multiple low energy photons can
be absorbed, via short-lived
“virtual states” (lifetime ~ 1 fs).
Can get to far-UV wavelengths
without “waste”
Spatial localization is excellent
(because of the high energy
needed, it can be confined to < 1
m3.)
Applications: primarily
bioanalytical microscopy
excited
state
virtual
state
ground
state
J. B. Shear, “Multiphoton Excited Fluoroescence in Bioanalytical Chemistry”, Anal. Chem., 71, 598A-605A (1999).
Applications of Fluorescence
Applications in forensics: trace level analysis of specific
small molecules
Example: LSD (lysergic acid diethylamide) spectrum
obtained with a Fourier-transform instrument and a
microscope, but with no derivitization
M. Fisher, V. Bulatov, I. Schechter, “Fast analysis of narcotic drugs by optical chemical imaging”, J. Luminesc.. 2003, 102–103, 194–200.
Applications of Fluorescence
Applications in biochemistry:
analysis of proteins, enyzmes,
anything that can be tagged
with a fluorophore
In some cases, an externally-
introduced label can be
avoided.
In proteins, the tryptophan
(Trp), tyrosine (Tyr), and
phenylalanine (Phe) residues
are naturally UV-fluorescent
– Example: single -galactosidase
molecules from Escherichia coli
(Ec Gal)
– 1-photon excitation at 266 nm
Q. Li and S. Seeger, “Label-Free Detection of Single Protein Molecules Using Deep UV Fluorescence Lifetime Microscopy”. Anal. Chem. 2006, 78, 2732-2737.
Drug Discovery Applications
The inhibition of cytochrome P450 (CYP)
enzyme is an indicator of potential drugdrug interactions and drug toxicity.
Assays are needed to screen thousands
of compounds for CYP inhibition.
Fluorescence assays (usually performed
using plate readers) are widely used :
– Select a fluorogenic substrate – a poorlyfluorescent molecule that when metabolized
by CYP becomes fluorescent.
– Mix the substrate, a CYP isozyme, and the
candidate drug molecule and incubate.
– If fluorescence is reduced, the candidate is
interfering with the fluorogenic substrate’s
metabolism, and thus is a CYP inhibitor.
Image from:
http://www.biotek.com/fluorescencemicroplate-reader-a.htm
Image from J. La kowicz, “Principles of Fluorescence
Spectroscopy”, 3rd Ed., Springer, 2006, pg. 30.
E. H. Kerns and L. Di, “Drug-Like Properties: Concepts, Structure Design and Methods”, Academic Press, 2008, pg. 197-206.
Fluorescence Recovery after Photo-Bleaching
Fluorescence Recovery After Photo-bleaching (FRAP),
first reported in 1974, is a technique for measuring
motion and diffusion
– FRAP can be applied at a microscopic level.
– FRAP is commonly applied to microscopically heterogeneous systems
A high power laser first bleaches an area of the sample,
after which the recovery of fluorescence is monitored with
the low power laser
– Can also use a single laser that is attenuated with a Pockel’s cell
Applications of FRAP have included:
– Biological systems
– Diffusion in polymers
– Solvation in adsorbed layers on chromatographic surfaces
– Curing of epoxy resins
J. M. Kovaleski and M. J. Wirth, Anal. Chem. 69, 600A (1997).
Fluorescence Recovery After Photo-bleaching
FRAP starts with fluorescence (left-hand image):
A periodic pattern is photobleached with a high power
laser (middle image)
The recovery of the fluorescence is monitored via a
low power laser (right-hand image)
J. M. Kovaleski and M. J. Wirth, Anal. Chem. 69, 600A (1997).
B. A. Smith and H. M. McConnell, Proc. Natl. Acad. Sci. USA. 75, 2759 (1978).
Fluorescence Recovery After Photo-bleaching
In spot photobleaching, a spot is bleached, and its
subsequent recovery is predicted by:
1/ 2
2
4D
1/2 is the time for the fluorescence to
recover 1/2 of its intensity
is the diameter of the spot
D is the diffusion coefficient
depends on the initial amount of
fluorophor bleached
Periodic pattern photobleaching (depicted on previous
slide) eliminates dependence, is more flexible and
accurate. Relies on Ronchi rulings or holography
Recovery is given by a simpler equation:
d2
1/ 2 2
4 D
FRAP requires a fluorophore: an organic fluorescent
molecule that is photo-bleached (ex. rhodopsin)
J. M. Kovaleski and M. J. Wirth, Anal. Chem. 69, 600A (1997).
D. E. Koppel, D. Axelrod, J. Schlessinger, E. Elson, and W. W. Webb, Biophys. J. 16, 1315 (1976).
Fluorescence At Sea
NH4+ can be detected at
low levels in seawater (for
environmental monitoring)
using several reactions:
Indophenol blue (Berthelot
reaction), LOD = 0.6 M
Ammonia electrode, LOD =
0.2 M
o-phthaldialdehyde (OPA)
with sulfite, LOD ~ nM, plus
fast kinetics (several
minutes)
OPA-sulfite-NH4+ run with a
flow-injection system for
shipboard use (LOD = 1.1
nM in lab)
N. Amornthammarong and J. Z. Zhang, Anal. Chem. 80, 1019-1026 (2008).
Fluorescence At Sea
The result:
a M-level “map” of NH4+ off the coast of
Florida (shows water quality – too much NH4+ is toxic)
N. Amornthammarong and J. Z. Zhang, Anal. Chem. 80, 1019-1026 (2008).
Fluorescence in Solids
Solid powders can be analyzed using powder packed
Intensity x 107 (counts per second/mA)
behind a quartz cover slip and held in a vertical position.
Front-facing (but 30 offset) geometries are generally
used instead of right angles for maximum signal because
the sample cannot emit in all directions.
Example: FL excitation and emission spectra of
crystalline diflunisal (Form 1):
Emission scan with
excitation at 340 nm
Excitation scan monitoring
emission at 465 nm
1.8
1.6
1.4
1.2
1.0
0.8
0.6
0.4
0.2
0.0
240
260
280
300
320
340
360
380
400
420
440
Emission wavelength (nm)
460
480
500
520
540
Molecular Phosphorescence
Phosphorescence – often used as a
complementary technique to fluorescence.
– If a molecule won’t fluorescence, sometimes
it will phosphoresce
excitation
fluorescence
phosphorescence
– Phosphorescence is generally longer
wavelength that fluorescence
Some phosphorimeters are “pulsed-source”,
which allows for time-resolution of excited
states (which have lifetimes covering a few
orders of magnitude).
– Pulsed sources also help avoid the
interference of Rayleigh scattering or
fluorescence.
Instrumentation similar to fluorescence, but
with cooling dewars and acquisition delays
wavelength
Note that the wavelength
difference between F and P
can be used to measure the
energy difference between
singlet and triplet states
Phosphorescence Studies
Room-temperature Phosphorescence (RTP)
– Phosphorescence is performed at low temperatures (77K) to avoid
“collisional deactivation” (molecules hitting each other), which causes
quenching of phosphorescence signal
By absorbing molecules onto a substrate, and evaporating the solvent,
the phosphorescence of the molecules can be studied without the need
for low temperatures
By trapping molecules within micelles (and staying in solution), the same
effect can be achieved
Applications:
– nucleic acids, amino acids, enzymes, pesticides, petroleum products, and
many more
For more details, see: R. J. Hurtubise, Phosphorimetry: Theory, Instrumentation, and Applications, Chap. 3, New York, VCH 1990.
Chemiluminescence (CL)
A chemical reaction that yields an electronically excited
species that emits light as it returns to ground state.
In its simplest form:
A + B C* C + h
The radiant intensity (ICL) depends on the rate of the
chemical reaction and the quantum yield:
ICL = CL (dC/dt) = EX EM (dC/dt)
excited states per
molecule reacted
photons per
excited states
Chemiluminescence of Gases
CL reactions can be used to quantitatively analyze gases
Example: Determination of nitrogen monoxide to 1 ppb
levels (for pollution analysis in atmospheric gases)
Figure from: http://www.shu.ac.uk/schools/sci/chem/tutorials/molspec/lumin1.htm
Chemiluminescence: Luminol Reactions
Luminol, a molecule that when oxidized can
Luminol reaction
(from Wikimedia commons)
do many things…
Representative uses of luminol:
– Detecting hydrogen peroxide in seawater1
(indicator of photoactivity)1
– Visualizing bloodstains – reaction catalyzed by
haemoglobin2
– Detecting nitric oxide3
NH2
NH2
O
NH
+
O
O-
oxidizing
agent
+
-
O
NH
O
O
luminol
1. D. Price, P. J. Worsfold, and R. F. C. Mantoura, Anal. Chim. Acta, 1994, 298, 121.
2. R. Saferstein, Criminalistics: An Introduction to Forensic Science, Prentice Hall, 1998.
3. J. K Robinson, M. J. Bollinger and J. W. Birks, Anal. Chem., 1999, 71, 5131.
See also http://www.deakin.edu.au/~swlewis/2000_CL_demo.PDF
hv
Applications of Chemiluminescence
Detection of arsenic in water:
– Convert As(III) and As(V) to AsH3 via borohydride reduction
– pH < 1 converts both As(III) and As(V), pH 4-5 converts only
As(III)
– Reacts with O3 (generated from air), CL results at 460 nm
– CL detected via photomultiplier tube down to 0.05 g/L for 3 mL
– Portable, automated analyzer, 6 min per analysis
– See A. D. Idowu et al., Anal. Chem., 2006, 78, 7088-7097.
Chemiluminescence can be applied to fabricated
microarrays on a flow chip, allowing for patterned
biosensor applications:
– See e.g. Cheek et al., Anal. Chem., 2001, 73, 5777.
Electrochemiluminescence
Electrochemiluminescence
(ECL): species formed at
electrodes undergo
electron-transfer reactions
and produce light
– ECL converts electrical
energy into radiation
– This scheme shows both an
oxidation and a reduction
occuring at an electrode; in
most cases a co-reactant is
used so that only one
electrochemical step is
needed
M. M. Richter, Chem. Rev. 2004, 104, 3003-3036
Electrochemiluminescence
ECL luminophores, such as the Ru(bipy)32+ luminophore,
have been the basis of a wide variety of immunoassays
and DNA hybridization assays:
W. Miao and A. J. Bard, Anal. Chem. Rev. 2003, 75, 5825-5834.
Further Reading
Required:
L. B. McGown, K. Nithipatikom (2000): Molecular fluorescence and phosphorescence,
Appl. Spectrosc. Rev. 2000, 35, 353-393.
Optional:
J. Cazes, Ed. Ewing’s Analytical Instrumentation Handbook, 3rd Edition, Marcel Dekker,
2005, Chapter 6. (Note: this is an updated version of the McGown article above).
D. A. Skoog, F. J. Holler and S. R. Crouch, Principles of Instrumental Analysis, 6th
Edition, Brooks-Cole, 2006, Chapter 15.
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd Edition, Springer, 2006.
D. H. Williams and I. Fleming, Spectroscopic Methods in Organic Chemistry, McGraw-Hill
(1966).
S. Das et al. “Molecular Fluorescence, Phosphorescence, and Chemiluminescence
Spectrometry, Anal. Chem. 2012, 84, 597–625.
M. E. Dias-Garcia, et al., “The triplet state: Emerging applications of room temperature
phosphorescence spectroscopy,” Appl. Spectrosc. Rev., 2007, 42, 605–624.
http://www.horiba.com/us/en/scientific/products/fluorescencespectroscopy/tutorialswebinars/basic-principles-of-fluorescence-spectroscopy/