Unit 4 - Alkyl Halides
advertisement

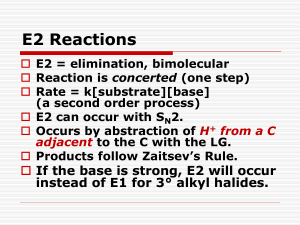
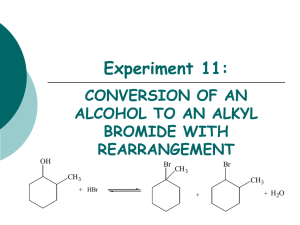
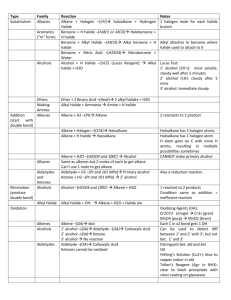
Unit 4 Alkyl Halides: Nucleophilic Substitution and Elimination Reactions Nomenclature of Alkyl Halides Apply the same rules you learned for the alkanes. CH3CH2Br IUPAC: Name the halogen as a substituent. bromoethane Common name: The alkyl group is named as the substituent. Ethyl bromide Nomenclature of Alkyl Halides 3-(iodomethyl)pentane I F 4-(2-fluoroethyl)heptane Nomenclature of Alkyl Halides An alkyl halide has a halogen bonded to an sp3 C. Methyl halides, CH3X: the sp3 C is bonded only to H atoms. CH3Br X denotes a halogen: F, Cl, Br, I. Primary (1°) halides, RCH2X: the sp3 C is bonded to one other C atom. CH3CH2Br Nomenclature of Alkyl Halides 2° halide methyl halide 1° halide 3° halide Nomenclature of Alkyl Halides A vicinyl dihalide has two halogens bonded to neighboring sp3 C atoms. A geminal dihalide has two halogens on the same sp3 C. Nomenclature of Alkyl Halides A allylic halogen is one that is bonded to the sp3 C atom immediately next to a double bond. FYI: The allylic position can be occupied by a substituent other than a halogen. IR Spectrum of an Alkene (for comparison) IR Spectrum of an Allylic Chloride The C-Cl str is 600-800 cm-1. Nomenclature of Other Halides A vinyl halide has a halogen bonded to an sp2 C of an alkene. An aryl halide has a halogen bonded to one of the sp2 C atoms of the aromatic ring. toluene chlorobenzene Nomenclature of Other Halides A benzylic halide has a halogen bonded to an sp3 C substituent of benzene. Nomenclature: Nitro, Phenyl A benzene ring as a substituent is called phenyl, -C6H5. Another substituent to know is the nitro group, -NO2. TNT trans-9-phenyl-2-methylundec-3-ene Uses of Alkyl Halides Solvents 1,1,1-trichloroethane and methylene chloride Starting materials for syntheses. Anesthetics Halothane Freons Pesticides DDT, lindane (lice), chlordane (termites) C-X is a Polar Bond Polar bonds have dipole moments which are proportional to both partial charge and bond length. Partial charge increases with electronegativity: I<Br<Cl<F. Bond length increases down the group. These two trends are in opposite directions, and results in the C-Cl bond being the most polar, with C-F and CBr close behind. C-X is a Polar Bond E.N. bond length partial charge dipole moment F 4.0 1.38 0.23 1.51 D Cl 3.2 1.78 0.18 1.56 D Br 3.0 1.94 0.16 1.48 D I 2.7 2.14 0.13 1.29 D for comparison dipole moment C-H 0.3 D N-H 1.31 D O-H 1.53 D C=O 2.4 D C≡N 3.6 D Intermolecular Forces in Alkyl Halides The strongest intermolecular force in alkyl halides is the London force (instantaneous dipole-induced dipole). This force increases with molecular weight and with surface area. Since the C-X bond is polar, there will also be a contribution from the dipoledipole attraction. Physical Properties of Alkyl Halides - Boiling Points Here the surface area has more effect than the C-X dipole. Since atomic size increases down the halogen group, boiling points will, too, if the alkyl part of the compound is the same. For compounds with similar molecular weights, branched compounds will have lower boiling points than compounds with straight chains. Physical Properties of Alkyl Halides - Densities Alkyl fluorides are comparable to alkanes and are less dense than water. Alkyl chlorides with one Cl are less dense than water. Alkyl bromides and iodides are denser than water. Preparation of Alkyl Halides Allylic Bromination We will learn other ways to prepare alkyl halides in later units. Free-radical halogenations often give a mixture of products. Allylic bromination, a free-radical process, can be selective. Allylic Bromination Mechanism Initiation Propagation, step 1 hυ Br2 2Br• allylic free radical Allylic Bromination Mechanism The allylic free radical is stabilized by resonance and will form two products. Allylic Bromination Mechanism Propagation, step 2. + Br• + Br• Allylic Bromination - Overall Reaction NBS, n-bromosuccinimide, is often used as the source of bromine. That way, the amount of bromine is kept low…why? NBS, hv H2C=CH-CH2-CH3 H2C=CH-CHBr-CH3 + H2CBr-CH=CH2-CH3 Allylic Bromination - NBS O O N O Br + HBr Br2 + NH O Br2 is present in trace amounts in NBS (for initiation), and HBr is formed as soon as any products are formed, and so is available to produce more Br2.