色彩與環境化學
『從原子到宇宙』課程第七週
胡維平
國立中正大學化學暨生物化學系
11/01/2012
Colors and Molecular Spectra
鹵素
染料
硫晶體
PbMoO4
MnCO3
Chromium Compounds
Richard Megna/Fundamental Photographs
Chemistry of Vision
環境化學
The Future of the Earth ?
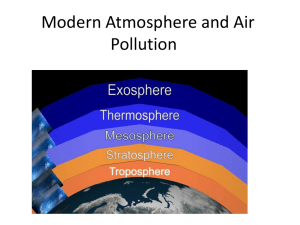
Properties of Our Atmosphere
2009, Prentice-Hall,
Solar Radiation
The atmosphere
is the first line of
defense against
radiation from
the Sun.
2009, Prentice-Hall,
Composition of the Atmosphere
• Because of the great
variation in atmospheric
conditions, the
composition of gases in
the atmosphere is not
uniform.
• Lighter gases tend to rise
to the top.
2009, Prentice-Hall,
Composition of the Atmosphere
• Near the Earth’s surface,
about 99% of the
atmosphere is composed
of nitrogen and oxygen.
• Oxygen has a much lower
bond enthalpy than
nitrogen, and is therefore
more reactive.
2009, Prentice-Hall,
Outer Atmosphere
• The Sun emits a
wide range of
wavelengths of
radiation.
• Remember that light
in the ultraviolet
region has enough
energy to break
chemical bonds.
2009, Prentice-Hall,
Ozone
• Ozone absorbs much of the radiation between 240
and 310 nm.
• It forms from reaction of molecular oxygen with
the oxygen atoms produced in the upper
atmosphere by photodissociation.
O + O2 O3
2009, Prentice-Hall,
Ozone Depletion
In 1974 Rowland and Molina discovered that chlorine from
chlorofluorocarbons (CFCs) may be depleting the supply of ozone in
the upper atmosphere by reacting with it.
2009, Prentice-Hall,
Chlorofluorocarbons
• CFCs were used for years as aerosol propellants and
refrigerants.
Freon 11: CCl3F, Freon 12: CCl2F2
• They are not water soluble and are quite unreactive.
• The C—Cl bond is easily broken, though, when the
molecule absorbs radiation with a wavelength
between 190 and 225 nm.
• The chlorine atoms formed react with ozone:
Cl + O3 ClO + O2
2009, Prentice-Hall, Inc.
Adopted from “UV Photolysis of ClOOCl and Ozone Hole” by Jim J. Lin, Andrew F
Chen, Yuan T. Lee, Chemistry, An Asian Journal, submitted.
Air Pollution
•
Two main sources:
Transportation
Production of
electricity
• Combustion of petroleum
produces CO, CO2, NO,
and NO2, along with
unburned molecules from
petroleum.
16
Nitrogen Oxides (Due to Cars and Trucks)
•
•
•
At high temperatures, N2 and O2 react to form NO, which
oxidizes to NO2.
The NO2 breaks up into nitric oxide and free oxygen atoms.
Oxygen atoms combine with O2 to form ozone (O3).
Radiant
energy
NO2 (g )
NO(g ) O(g )
O(g ) O2 (g )
O3 (g )
17
Sulfur Oxides
(Due to Burning Coal for Electricity)
•
•
Sulfur produces SO2 when burned.
SO2 oxidizes into SO3, which combines with
water droplets in the air to form sulfuric acid.
S(in coal) O2 (g )
SO2 (g )
2SO2 (g ) O2 (g )
2SO3 (g )
SO3 (g ) H2O(l )
H2SO4 (aq )
Copyright © Cengage Learning.
All rights reserved
18
酸雨
• High acidity in rainfall causes corrosion
in building materials.
• Marble and limestone (calcium carbonate)
react with the acid; structures made from
them erode.
Sulfur
• Sulfur dioxide is a by-product of the burning of coal or oil.
• It reacts with moisture in the air to form sulfuric acid.
• It is primarily responsible for acid rain.
2009, Prentice-Hall,
煤除硫化
• SO2 can be removed by
injecting powdered
limestone which is
converted to calcium
oxide.
• The CaO reacts with
SO2 to form a
precipitate of calcium
sulfite.
2009, Prentice-Hall,
Carbon Monoxide
• Carbon monoxide binds
preferentially to the iron
in red blood cells.
• Exposure to significant
amount of CO can lower
O2 levels to the point
that loss of
consciousness and death
can result.
2009, Prentice-Hall,
Photochemical Smog
• These nitrogen oxides are just some components of
photochemical smog.
• Ozone, carbon monoxide, and hydrocarbons also contribute to air
pollution that causes severe respiratory problems in many people.
• government emission standards for automobile exhaust have
become continually more stringent.
Hybrid Car
Water Vapor and Carbon Dioxide
• Gases in the atmosphere form an
insulating blanket that causes the
Earth’s thermal consistency.
• Two of the most important such gases
are carbon dioxide and water vapor.
Water Vapor and Carbon Dioxide
• This blanketing effect is
known as the “greenhouse
effect.”
• Water vapor, with its high
specific heat, is a major
factor in this moderating
effect.
• But increasing levels of CO2
in the atmosphere may be
causing an unnatural increase
in atmospheric temperatures.
2009, Prentice-Hall,
Chemical Processing Plants
Solvents such as supercritical CO2 are
great “green” alternatives.
Simon Fraser/SPL #S4644E
什麼是優養化 (Algal bloom)?
「優養化」是指水中的氮、磷等營養元素不斷增加 (PO42-,
NO3-),造成水中藻類大量繁殖,藻類耗用水中溶氧量,造成
水棲生物、魚類、蝦蟹缺氧、死亡,動物屍體又會惡化水體,
惡性循環下,「優養化」就不斷加遽。
自然界中,氮、磷是稀有的成分,但是近年水庫上游因人為開
發,在山坡地種植蔬果,噴撒農藥、肥料,或是人為排放的廢
水、洗衣粉,都含氮、磷,才加速水庫優養化。