Polymer Synthesis CHEM 421
advertisement

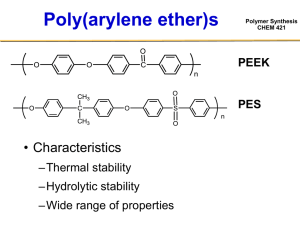
Polymer Synthesis CHEM 421 • Odian Book 2-12 Common Engineering Thermosets (Not elastomers) Polymer Synthesis CHEM 421 • Bismaleimides Step & Chain Growth • Epoxies Step & Chain Growth • Phenol / Formaldehyde Step Growth • Sheet Molding Compound Chain Growth • Polyurethanes** **Also thermoplastics Step Growth Epoxy Systems Polymer Synthesis CHEM 421 f = 2 “Tube A” f = 4 “Tube B” Mechanism Network Epoxy Systems • Advantageous Properties of epoxies – High chemical and solvent resistance – Outstanding adhesion to many substrates – Good impact resistance – Good electrical properties Polymer Synthesis CHEM 421 Epoxy Systems Polymer Synthesis CHEM 421 f = 2 “Tube A” f = 4 “Tube B” Network Epoxy Systems Polymer Synthesis CHEM 421 Diglycidal Ether of Bisphenol-A (DGEBA) Epichlorohydrin: Background Chlorine intensive - 4 atoms of chlorine/epi Yields: - chlorination: 82% - HOCl and closure: 92% Byproducts: - chlorinated organics Hydraulic load: - 47 lbs water/lb of epi Polymer Synthesis CHEM 421 Epoxy Systems <Mn> ≈ 15,000 – 20,000 g/mol Viscous liquid to solid Polymer Synthesis CHEM 421 Polyurethanes and Polyureas • Thermoplastics • Thermosets Polymer Synthesis CHEM 421 Important Starting Materials for Polyurethanes Polymer Synthesis CHEM 421 • Diisocyantes • Polymeric Glycols (aka polyols) –MW < 3,000 g/mol • Chain extenders • Catalysts –Trialkyl tin acetate –Dialkyl tin diacetate Relative rates 30,000x Diisocyanates • Diphenylmethane diisocyanate (MDI) • Toluene diisocyanate (TDI) • Dicyclohexylmethane diisocyanate (H-MDI) • Hexamethylene diisocyanate (HDI) • Cycloaliphatics Polymer Synthesis CHEM 421 Diisocyanates Polymer Synthesis CHEM 421 • Phosgenation O H N C Cl N C O + HCl Chain Extenders • For urethanes • For ureas Polymer Synthesis CHEM 421 Polyurethane Fibers Polymer Synthesis CHEM 421 Excess Pre-polymer Chain extenders Sheet Molding Compound Polymer Synthesis CHEM 421 Sheet Molding Compound Polymer Synthesis CHEM 421 Phenol Formaldehyde Resins Polymer Synthesis CHEM 421 • 1872 – Invented by Bayer • 1907 – First patent and commercial process by Baekeland • Success: First wholly synthetic polymer used commercially Phenol Formaldehyde Resins • Excellent thermal stability • High char yield • Low smoke generation • Low smoke toxicity Polymer Synthesis CHEM 421 Base-Catalyzed PhenolFormaldehyde Resins (Resols) • Base catalyzed • Excess formaldehyde • Resols cure with heat alone Polymer Synthesis CHEM 421 Base-Catalyzed PhenolFormaldehyde Resins (Resols) Polymer Synthesis CHEM 421 Base-Catalyzed PhenolFormaldehyde Resins (Resols) • Excess formaldehyde • Resols cure with heat alone Polymer Synthesis CHEM 421 Polymer Synthesis CHEM 421 Base-Catalyzed PhenolFormaldehyde Resins (Resols) • Cure of resole prepolymer proceeds under neutral or acidic conditions and at elevated temperature. • Crosslinking occurs via the continued formation of methylene links and the formation of dibenzyl ether linkages. • Higher temperatures favor the formation of methylene bridges • Both are condensation reactions and produce water Polymer Synthesis CHEM 421 Acid-Catalyzed PhenolFormaldehyde Resins (Novolacs) Polymer Synthesis CHEM 421 Acid-Catalyzed PhenolFormaldehyde Resins (Novolacs) • • • • • • Acid Catalyzed Excess phenol No hydroxy methyl groups Tg = 40 C MW = 1 – 3000 g/mol Require second additive for cure – Hexamethylene tetraamine Polymer Synthesis CHEM 421