Chapter13
advertisement

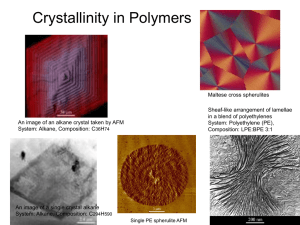
ENS 205 Materials Science I Chapter 13: Polymers Basics I vinyl chloride Poly (vinyl chloride) A polymer is a substance composed of molecules with large molecular mass composed of repeating structural units (called monomers), connected by covalent chemical bonds. Basics II linear polymer crosslinked polymer star polymer branched polymer dendrimer Polymer Structure I Why most? Because a few types of polymers may do under certain circumstances (we will see how) amorphous polymer semi-crystalline polymer MOST CRYSTALLINE POLYMERS ARE NOT ENTIRELY CRYSTALLINE !!! They are either amorphous or semi-crystalline. The degree of crystallinity varies (From 0 to 90-95 %). Polymer Structure II Shish-kebab morphology SEM image of a chain having shish-kebab morphology SEM image of a spherulite Polymer Structure III Crystallinity of polymers is affected by: All about the chain properties Chemical structure Stereochemistry Molecular weight Temperature Processing conditions Hmm… External effects Polymer Structure IV STEREOCHEMISTRY (Tacticity) This is the white, plastic coffee cup used everywhere like Fassane. Strafor kopuk Is polystyrene flat like this? Absolutely NO! Polymer Structure V STEREOCHEMISTRY (Tacticity) In the previous picture you see all the phenyl groups are located on the same side of the polymer chain. But they don't have to be this way. To illustrate let's look at a chain of polystyrene from above. You can see that the pendant phenyl groups can be either on the right or left side of the chain. All phenyl groups on the same side Phenyl groups on alternating sides Phenyl groups distributed randomly Polymer Structure V STEREOCHEMISTRY (Tacticity) The question is, how tacticity helps crystallinity CRYSTALLINITY ↔ LONG RANGE ORDER ↔ PACKING Syndiotactic polystyrene: Highly crystalline Atactic polystyrene: Highly amorphous Similarly, a linear polymer can pack well, whereas a branched isomer cannot Highly crystalline Highly amorphous Polymer Structure VI Intermolecular forces and crystallinity (Aromatic ring stacking) Polymer Structure VII • Fibers – Polymers with regular structure can align themselves very closely for effective utilization of the secondary intermolecular bonding forces. – Already stretched out fully, up to 95% crystallinity – High symmetry, high intermolecular forces. – Characterized by high modulus, high tensile strength, and moderate extensibilities Can you stretch this structure??? High density polyethylene Polypropylene Nylon Polyester Kevlar and Nomex Polyacrylonitrile Cellulose Polyurethanes Polymer Structure VIII • Elastomers (rubbers) – Polymers with irregular structure, weak intermolecular attractive forces and flexible chains. – Can undergo local mobility, but gross mobility of chains is restricted. – Characterized by high extensibility, low initial modulus in tension but they stifen when strecthed. stretch leave ENTROPY WORK! Polymer Structure IX • Plastics – Fall between the elastomers and fibers. However there is no exact boundary – Harder to stretch than elastomers (Because of crystalline regions?). But preserve their shape when stretched unlike elastomers (Strain induced crystallization, stiff chains) – They are pliable, that is, they can be shaped and molded easily – Thermoplastics: Melt when heated and can be melted again after cooling – Thermosets: Undergoes crosslinking when heated, so does not melt again, decomposes if heated further – Flexible plastics: Plastics above their Tg. Flexible, soft – Rigid plastics: Plastics below their glass transition temperature (Tg). Brittle, hard What are Tg, crosslinking and “melting for polymers” ? Polymer Structure X Glass transition temperature (Tg) • Different polymers have different segments on their backbones. The ease of movement of these segments (portions of the chain) depends on the structure, physical environment of the chain etc. of the segment. • Any movement of these segments require energy which is kinetic in this case, right? Then each different polymer would have different energy requirement for the movement of these segments (different polymer = different structure, different physical environment of the chain etc). • Below glass transition temperature, these segments do not have sufficient energy to move. So, if you apply some stress, say if you try to bend a polymer which is below its Tg then the segments won’t be able to move into new positions to relieve the stress which you have placed on them; which will make the polymer brittle. Above Tg they would, so they would be flexible. • Always keep this in mind: Tg IS A PROPERTY RELATED WITH THE AMORPHOUS REGIONS OF THE POLYMER, NOT CRYSTALLINE! • So it should now be obvious that elastomers are elastomers above their Tg. Below, they are not elastomers, they are glassy, because they are not flexible anymore (Remember my experiment with rubber glove and liquid nitrogen during the lecture). Polymer Structure XI Melting • Melting is a transition which occurs in crystalline polymers. • Melting happens when the polymer chains fall out of their crystal structures, and become a disordered liquid. • Always keep this in mind: MELTING IS A PROPERTY RELATED WITH THE CRYSTALLINE REGIONS OF THE POLYMER! So do you think you can melt atactic polystyrene? (No, because it is not crystalline) Question: What if I see both melting and glass transition in the differential scanning calorimeter (DSC) spectrum of a polymer sample??? It is absolutely OK. Remember, most polymers are semicrystalline, i.e. have both amorphous and crystalline regions Polymer Structure XII Crosslinking Poly (1,4-butadiene) Synthetic rubber Crosslinking with sulphur (vulcanization) This is the tire of your car Thus, it is possible to produce elastomers via crosslinking! (In fact, it is not only possible but also the very common way of making elastomers, i.e. rubber) Mechanical properties Now, I am sure that you can rationale the mechanical behavior of various types of polymers shown in this image. How to make polymers Step growth (Condensations) Chain growth (Addition) Initiator Monomer Monomer (ethylene) Monomer H2O out Monomer • Free radical • Ionic Polyethylene This is the plastic bag given in the supermarket Ring opening (PET) This is the bottle of your 1 lt coke This is the breast implant of your favorite female model Poly(dimethyl siloxane) Molecular weight • Not all of the chains of a polymer are of same length. Their size differ most of the time. • Remember: A chain is a polymer molecule (in fact the chain is the polymer itself), so the molecular weight of a polymer should in fact be the molar mass of a single polymer chain. However, since chains have different lengths (that is why we call polymers as polydisperse) we can only talk about averages • Number average molecular weight = • Weight average molecular weight = • Polydispersity index = Mw / Mn Ni is the number of chains having molecular weights Mi Configuration (Chain Structure) Copolymer (repeating units are more than one monomer type) homopolymer (repeating unit is always same monomer) Examples Examples Mechanical Testing Temperature Dependence of Deformation Processing Injection molding: http://www.bpf.co.uk/downloads/files/InjectionMoulding.swf Blow molding: http://www.pct.edu/prep/bm.htm http://www.youtube.com/watch?v=vSabFFQUr9E Compression molding: http://www.ticona.com/index/tech/processing/compression_molding.htm